Figure 1. Rational design of TEL-x-MM and semi-batch synthesis of TEL-x-BASP.
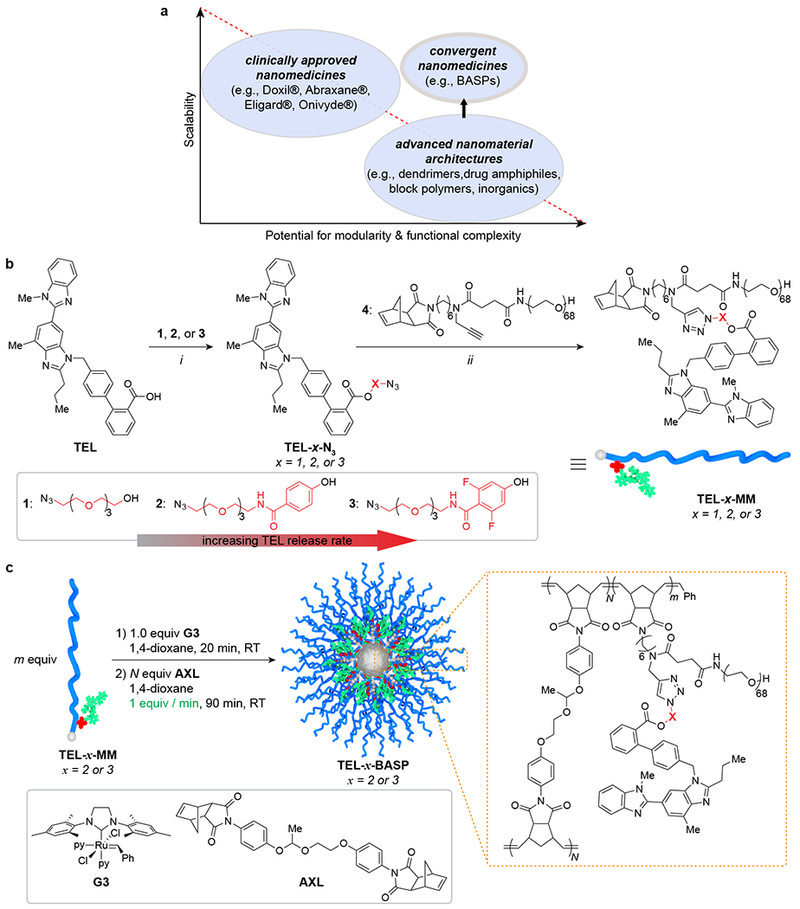
a. Diagram depicting the inverse relationship between scalability and potential for modularity and functional complexity in carriers for drug delivery. Convergent macromolecular prodrugs can potentially satisfy both demands. b. Synthesis of telmisartan macromonomers (TEL-MM) via esterification and CuAAC: i: EDC·HCl, DMAP, CH2Cl2, 16 h, 73% - 93%; ii: CuOAc, CH2Cl2, 1 h, 43% - 62%. Ester linkages between TEL and polymer increase in lability due to stronger electron-withdrawing groups (left to right). c. BASP synthesis via polymerization of TEL-x-MM (1) followed by semi-batch addition of AXL to induce crosslinking (2).
