Figure 5.
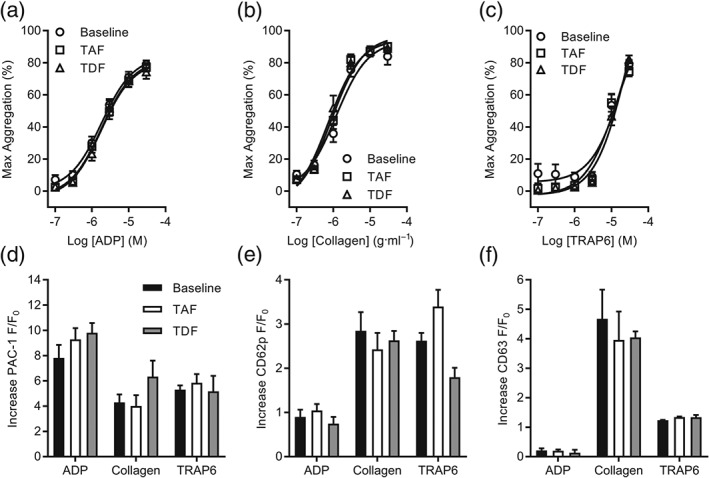
Steady‐state plasma concentrations of TAF or TDF in healthy subjects do not alter ex vivo platelet activation. (a–c) Platelet aggregation was monitored using PRP in a microplate assay. PRP was collected from healthy subjects enrolled on a Phase I clinical trial at predetermined timepoints: predose on Day 1 (baseline) and postdose following 28 days of once‐daily TAF (25 mg) or TDF (300 mg). Platelet aggregation was assessed in response to rising concentrations of ADP (a), collagen (b), or TRAP6 (c), and the maximum response is reported. (d–f) Flow cytometry was used to assess real‐time changes of platelet activation markers. PRP was collected from healthy subjects enrolled on a Phase I clinical trial at predetermined timepoints: predose on Day 1 (baseline) and postdose following 28 days of once‐daily TAF (25 mg) or TDF (300 mg). Activation of integrin αIIbβ3 (a) and surface expression of CD62P (b) and CD63 (c) were monitored following stimulation by ADP (10 μM), collagen (10 μg·ml−1), or TRAP6 (10 μM). Increases of fluorescence are reported, and intrasubject analysis was performed. Data are representative of 20 (a–c) and four (d–f) subjects enrolled on the trial. In (a‐c), no significant effects; two‐way ANOVA
