Figure 2.
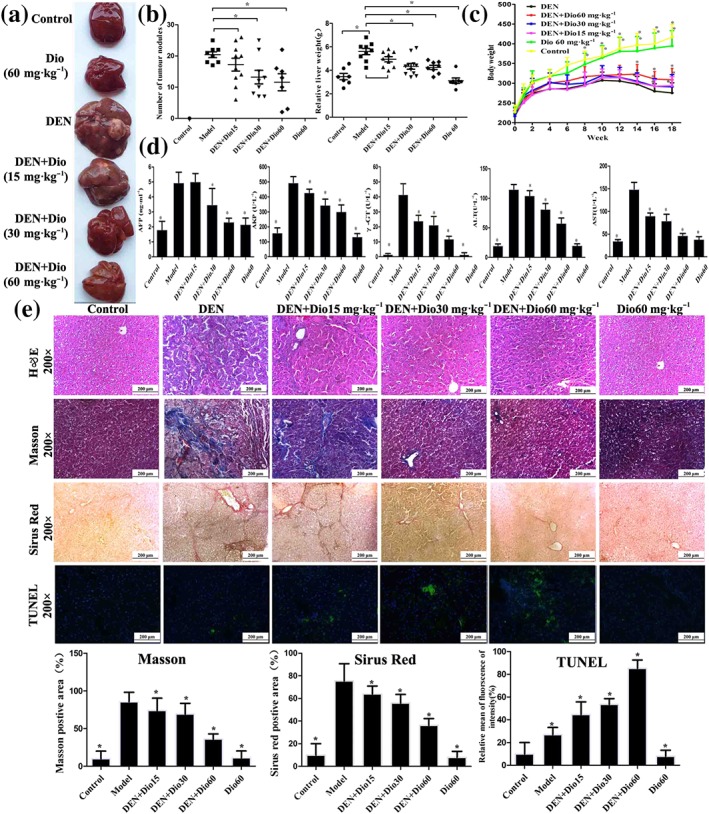
Effects of dioscin on primary liver cancer induced by DEN in rats. (a–b) Effects of dioscin on hepatic morphology of rats induced by DEN. The animals were randomly distributed into control group, model group (DEN), DEN+dioscin (60, 30, and 15 mg·kg−1) groups and dioscin group (60 mg·kg−1). The rats in model and DEN+dioscin groups received 0.01% DEN for 18 weeks, and other animals were given PBS. (c) Effects of dioscin on body weights of rats treated with DEN. The body weights of rats were measured once a week during the process. (d) Effects of dioscin on the serum levels of AFP, ALT, AST, ALP, and γ‐GT of rats induced by DEN. After 18 weeks of DEN administration, blood samples were collected and then the serum levels of AFP, ALT, AST, ALP, and GT were detected. (e) Effects of dioscin on primary liver cancer based on H&E, Masson, Sirius red, and TUNEL staining. All data are expressed as mean ± SD (n = 8). *P < 0.05 versus DEN model group
