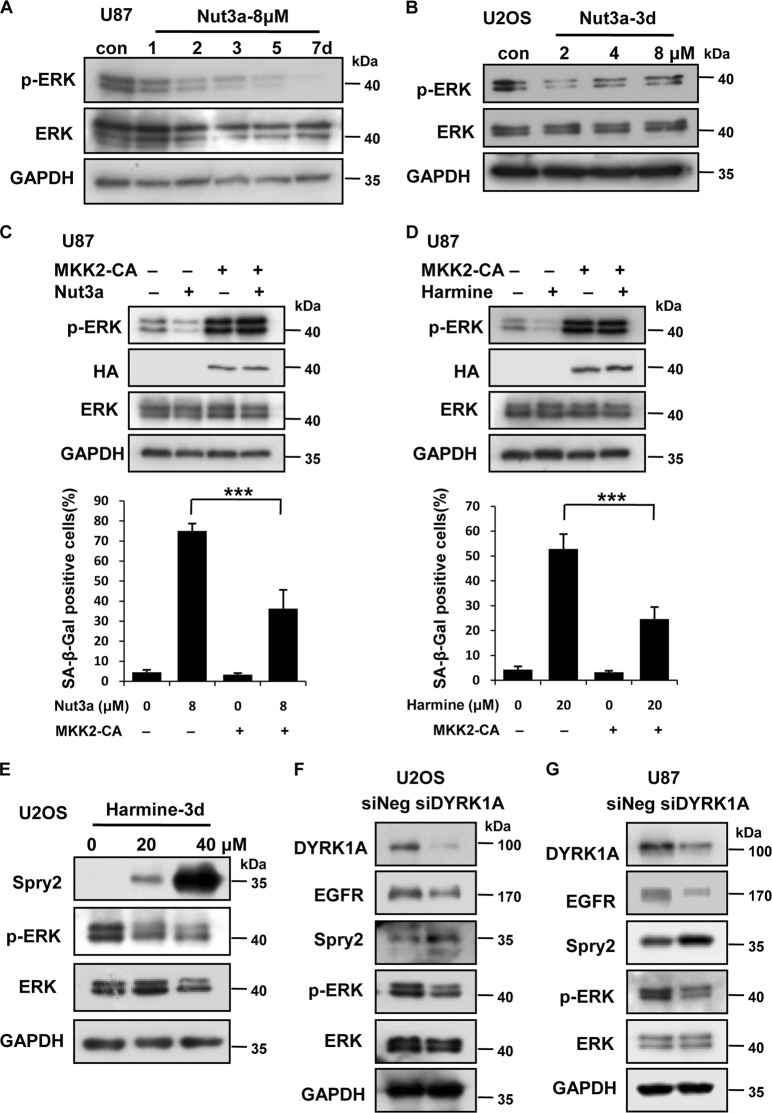
Fig. 4. Downregulation of the EGFR-ERK signaling pathway mediates the induction of senescence by p53 activation.
a, b The protein levels of phosaphorylated (p)-ERK and ERK in U87 and U2OS cells were measured by western blot. U87 cells were treated with the indicated time of 8 μM Nut3a, and U2OS cells were treated with the indicated concentration of Nut3a for 3 days. c Overexpression of MKK2 attenuates Nut3a-induced senescence. MKK2-CA expression vectors were transfected into U87 cells using Lipofectamine 2000. After expression for 48 h, add media with hygromycin B to get a good stably transfected cells. Then, the cells were treated with 8 μM Nut3a for 72 h. Cell extracts were examined by western blot for the determination of p-ERK, ERK, and HA-MKK2-CA protein levels. Bottom, stable MKK2-CA expression cells were treated with 8 μM Nut3a for 3 days. Cell senescence was examined by senescence-associated β-galactosidase (SA-β-gal) activity analysis. d The MKK2-CA expression cells were treated with 20 μM harmine for 72 h. Cell extracts were examined by western blot for the determination of p-ERK, ERK, and HA-MKK2-CA protein levels. Bottom, stable MKK2-CA expression cells were treated with 20 μM harmine for 3 days. Cell senescence was examined by SA-β-gal activity analysis. e–g p-ERK is downregulated by the depletion or inhibition of dual-specificity tyrosine-phosphorylated and tyrosine-regulated kinase 1A (DYRK1A) in U2OS or U87 cells. c U2OS cells were treated with the indicated concentration of harmine for 72 h. p-ERK, ERK, and Spry2 were measured by western blot. d, e, U2OS and U87 cells were transfected with small interfering RNA (siRNA) specific to DYRK1A or negative oligo for 48 h. The protein levels of DYRK1A, epidermal growth factor receptor (EGFR), Spry2, p-ERK, and ERK were measured by western blot. *** p < 0.001 vs. control