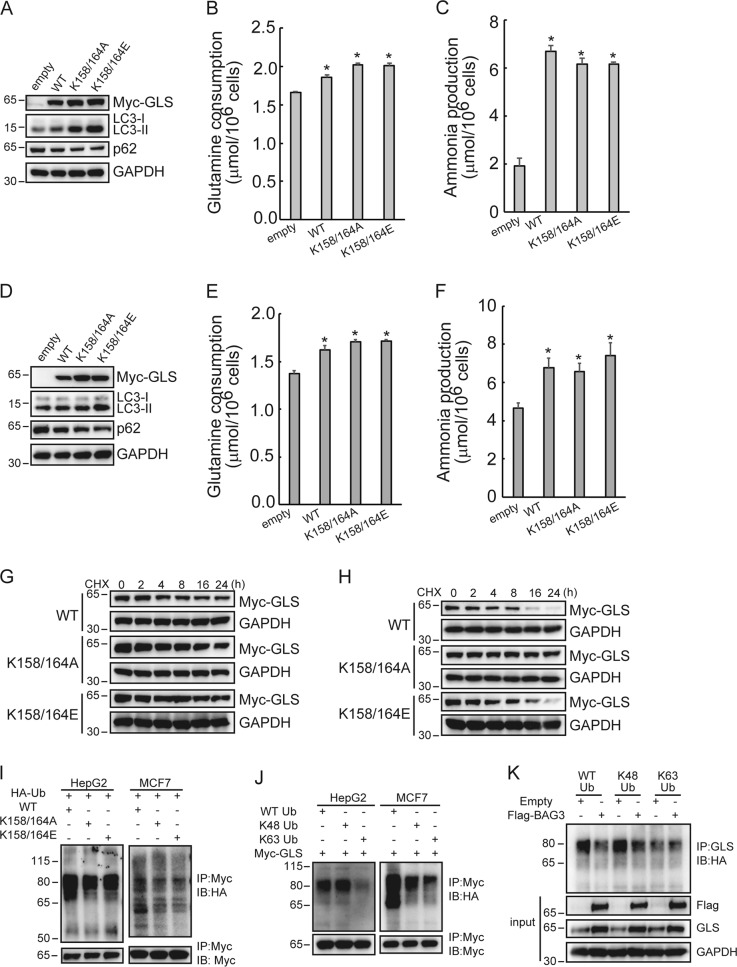
Fig. 6. Succinylation at Lys158 and Lys164 sites stabilizes GLS via suppression its Lys48-linked ubiquitination.
a–c HepG2 cells were infected with lentivirus containing empty, GLS (WT), GLS (K158/164 A) or GLS (K158/164E) construct. a Western blot analysis was performed using the indicated antibodies. Glutamine consumption (b) and ammonia production (c) were analyzed using the colorimetric method. d–f MCF7 cells were infected with lentivirus containing empty, GLS (WT), GLS (K158/164 A) or GLS (K158/164E) construct. d Western blot analysis was performed using the indicated antibodies. Glutamine consumption (e) and ammonia production (f) were analyzed using the colorimetric method. g and h The effect of overexpressing GLS (WT), GLS (K158/164 A) or GLS (K158/164E) on the half-life of GLS was investigated in indicated cells treated with cyclohexamide (CHX). i In vivo ubiquitination was performed by transfecting HepG2 or MCF7 cells with indicated expression plasmids. Cells were transfected with the indicated constructs for 36 h and incubated with MG132 for additional 4 h. Lysates were subjected to denaturing immunoprecipitation with an anti-Myc antibody and the levels of GLS (WT), GLS (K158/164 A) or GLS (K158/164E) ubiquitination were detected by immunoblotting with anti-HA antibody. j GLS polyubiquitination assay following transfection of HA-tagged ubiquitin (Ub) or its mutant forms K48 and K63. k Control or BAG3-overexpressing HepG2 cells were transfected with HA-Ub or its mutant forms K48 and K63, and analyzed by immunoprecipitation with an anti-Myc antibody and immunoblotting with the indicated antibodies. *P < 0.05. Error bars indicate means ± SD