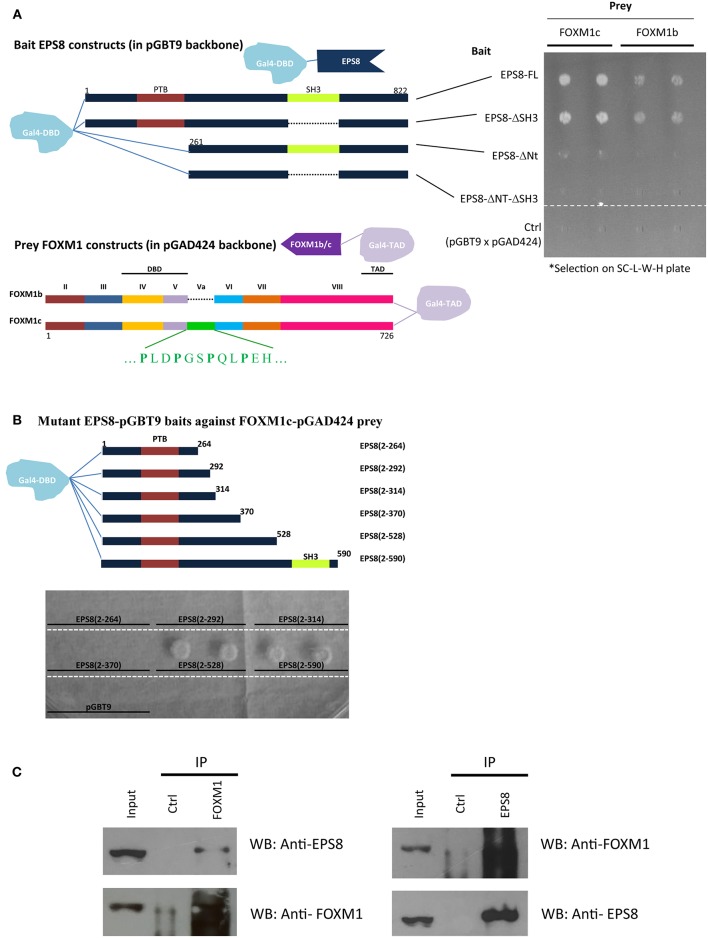
Figure 1.
Interaction of EPS8 and FOXM1 in yeast two-hybrid and IP assays. Yeast two-hybrid analysis was performed using the Matchmaker Gal4 Two-hybrid system (Clontech) on SC medium lacking leucine, tryptophan, and histidine (SC-L-W-H). Leucine and tryptophan dropout selected for the presence of the prey and bait plasmids whereas growth in histidine dropout plates indicated the strength of protein-protein interaction. (A) Interaction of EPS8 with full-length FOXM1 required the N-terminal region but not the SH3 domain of EPS8. EPS8 interacted with FOXM1c more strongly than FOXM1b. Exon Va (encoding an extra 15 amino acids) is present in FOXM1c but not FOXM1b. Deletion of the SH3 domain (EPS8-ΔSH3) from EPS8 did not affect the interaction but removal of the first 260 amino acids (EPS8-ΔNt), including the PTB domain, led to substantially decreased interaction, suggesting that the N-terminal region of EPS8 contains a critical FOXM1 interacting domain. As negative control, the parental constructs (pGBT9 for bait and pGAD424 for prey) were tested in parallel to rule out background effect. DBD, DNA binding domain; TAD, transcriptional activation domain. (B) Test of interaction of deletion constructs extending from the N-terminal region of EPS8 against full-length FOXM1 as prey. The N-terminal domain alone [EPS8(2-264)] was not sufficient for interaction and colony growth occurred when EPS8 extended beyond amino acid 370 in EPS8(2-528). Adding the SH3 domain by extending to amino acid 590 in EPS8(2-590) did not increase colony growth further, consistent with the SH3 domain being dispensable for interaction. (C) IP assays. HeLa cell lysates were subjected to IP with anti-FOXM1 antibody and control antibody (rabbit anti-ETS2). Immunoblot analysis using anti-EPS8 antibody indicated that endogenous EPS8 was pulled down. In the reverse IP experiment, FOXM1 could also be immunoprecipitated using anti-EPS8 antibody. Mouse anti-p21 antibody was used as the control antibody.