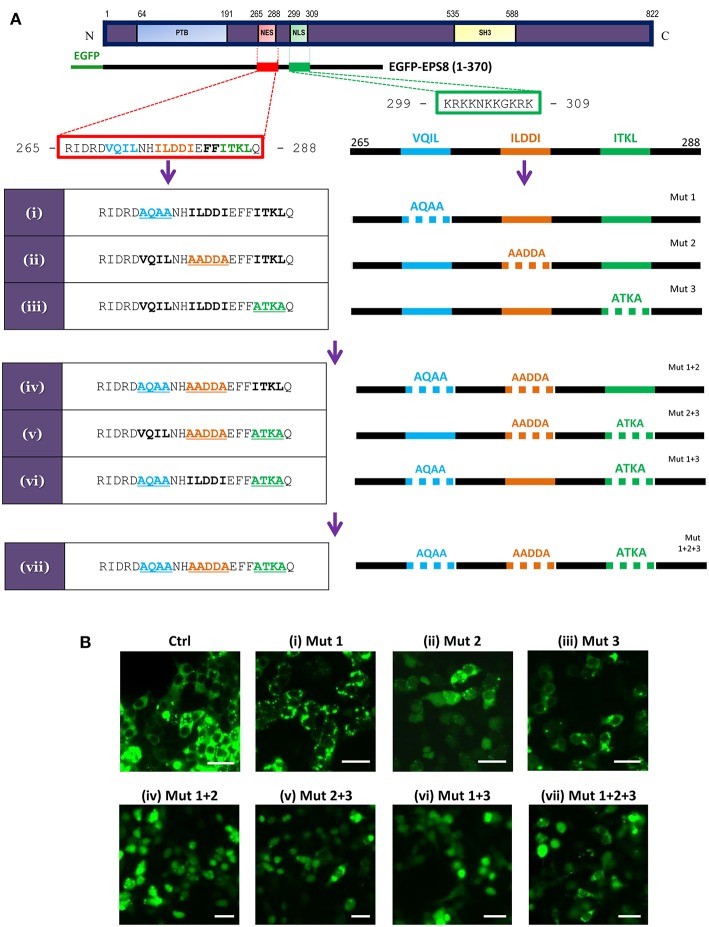
Figure 3.
EPS8 contains a functional NES. (A) Scheme for systematic alanine-scanning mutagenesis of a putative NES (amino acids 265-288: RIDRDVQILNHILDDIEFFITKLQ; hydrophobic residues in bold) using EGFP-EPS8(1-370) as backbone construct. A putative NLS (amino acids 299-309: KRKKNKKGKRK) is also shown. The three stretches of L/I-rich sequences (VQIL, ILDDI and ITKL) were singly, doubly or triply mutated to generate constructs (i) to (vii). (B) Assessing the nuclear export function of the putative NES. Wild-type control (Ctrl) and mutant EGFP-EPS8(1-370) constructs (i) to (vii) were individually transfected into HEK293K cells to assess the extent of nuclear localization of the various EPS8 fusion proteins. Fluorescence microscopy revealed that control (Ctrl) construct and constructs (i) and (iii) showed predominantly cytoplasmic signals whereas construct (ii) directed partial nuclear localization, suggesting that ILDDI is more critical for the nuclear export function. However, all double mutant constructs [(iv), (v) and (vi)] and the triple mutant construct (vii) showed significant nuclear localization, indicating that all three stretches of L/I-rich sequences are required for nuclear export. Scale bar, 10 μm.