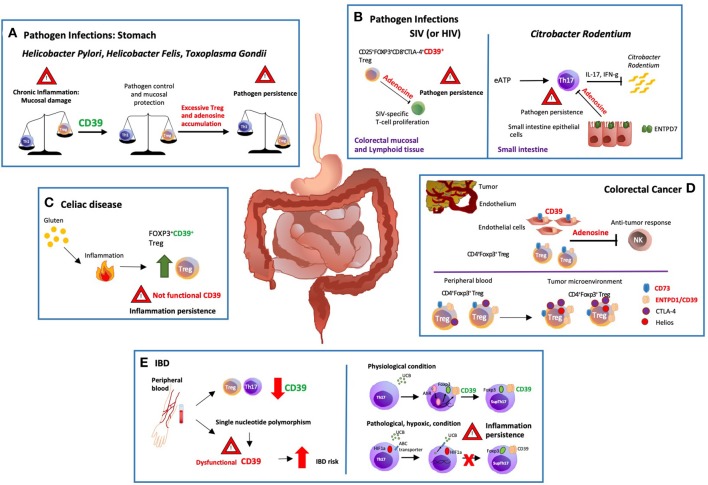
Figure 1.
Modulation of gastro-intestinal disease by ectonucleotidases. (A) There are potent immune responses to gastrointestinal bacterial and parasitic infections e.g., Helicobacter pylori, Helicobacter felis and Toxoplasma gondii. The balance between pro and anti-inflammatory signals controls the development and outcome of the disease. Protracted release of Th1-related cytokines contributes to the establishment of chronic inflammation that might ultimately result in peptic ulcer disease and gastric cancer. ENTPD1/CD39 expression by regulatory T-cells (Tregs) modulates Th-cell responses; however, excessive immune regulation can also lead to pathogen persistence. (B) In a macacus rhesus model of pathogenic simian immunodeficiency virus (SIV) infection, there is rapid expansion of CD25+FOXP3+CD8+CTLA-4+CD39+ Tregs, especially in the colorectal mucosal and lymphoid tissues. This event limits anti-viral responses by suppressing the proliferation of SIV-specific T-cells. Treg accumulation is also observed in HIV patients, implicating that therapeutic strategies controlling the expansion of CD25+FOXP3+CD8+CTLA-4+ CD39+ Tregs might effectively control HIV infection restoring the anti-viral response. (C) Celiac disease is a chronic inflammatory disorder triggered by aberrant immune responses to dietary gluten. Exposure to gluten induces protective accumulation of FOXP3+CD39+ Tregs that, however, display defective suppressive function, and do not adequately control aberrant inflammatory responses. (D) ENTPD1/CD39 and CD73 are the dominant ectonucleotidases expressed by tumor endothelial cells and Tregs. Extracellular adenosine generated by CD39+ Tregs isolated from the blood of cancer patients inhibits and suppresses anti-tumor responses. Further, the tumor microenvironment impacts the phenotype and function of local cells, substantially limiting immunotherapeutic strategies. In this regard, most of the colorectal cancer-infiltrating Tregs are Helios+ and express higher levels of ENTPD1/CD39 and cytotoxic T-lymphocyte antigen 4 (CTLA-4), when compared to peripheral blood and colon-derived counterparts. (E) Low levels of ENTPD1/CD39 expression by Tregs and Th17-cells are observed in the peripheral blood of patients with inflammatory bowel disease (IBD). Moreover, single nucleotide polymorphisms associated with low ENTPD1/CD39 mRNA levels, increase susceptibility to the disease. On the other hand, in vitro exposure to unconjugated bilirubin (UCB) results in increased levels of ENTPD1/CD39 and FOXP3 expression in Th17-cells derived from healthy individuals, through a mechanism mediated by aryl hydrocarbon receptor (AhR). However, Crohn's-derived Th17-cells remain refractory to UCB immunoregulation due to altered responses to hypoxia that inhibits AhR signaling by inducing ATP-binding cassette (ABC) transporters that promote UCB efflux out of Th17-cells.