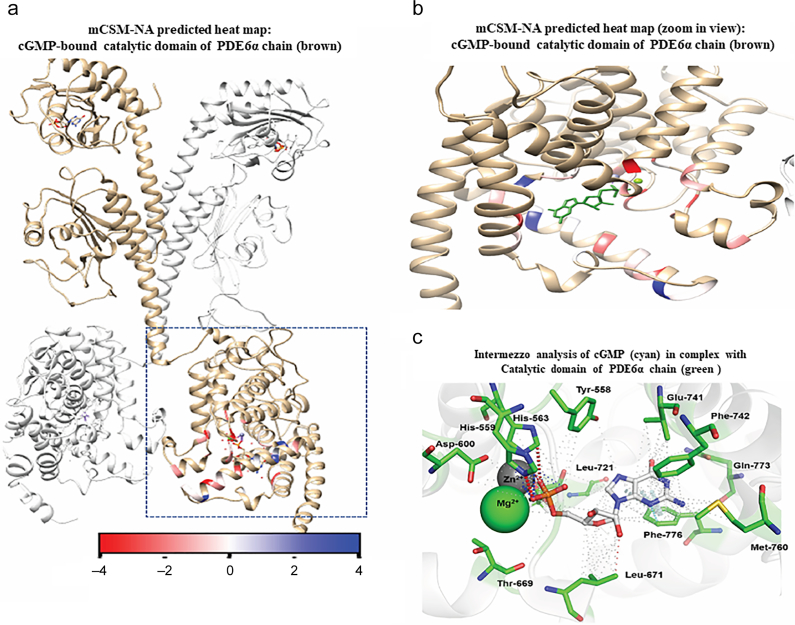
Fig. 6.
a) Heat map based on mCSM-NA predicted ΔΔG values showing the average stability changes upon mutation in cGMP binding residues of PDE6α catalytic domain. (b). Zoom in view illustrating the average effect of mutations in critical catalytic residues that binds cGMP molecule. Colored gradient scale shows the mutation effects; stabilizing (>0.00 kcal/mol) (blue), slightly destabilizing (≥−2.0 kcal/mol) (white) and highly destabilizing (<−2.00 kcal/mol) (highlighted in red) c) Intermezzo analysis depicts interatomic interactions between cGMP molecules and wild type residues of catalytic domain of PDE6 through non-covalent interactions. Red dotted lines indicate ionic bonds between His-563, His-559 and Zn2+ ion. Metal interactions involving Zn2+ ion and cGMP molecule are shown in blue color. Weak hydrogen bonds are shown in small red dotted lines. π-π stacking interaction and aromatic interaction of Phe-776 with the cGMP molecule within the catalytic pocket are shown in white and cyan colored dotted lines respectively. Other important catalytic residues i.e. Tyr-558, Asp-600, Thr-669, Leu-671, Glu-741, Phe-742, Met-760, Gln-773 are shown to have undefined interaction with the cGMP molecule depicted in grey small dotted lines.