Figure 6.
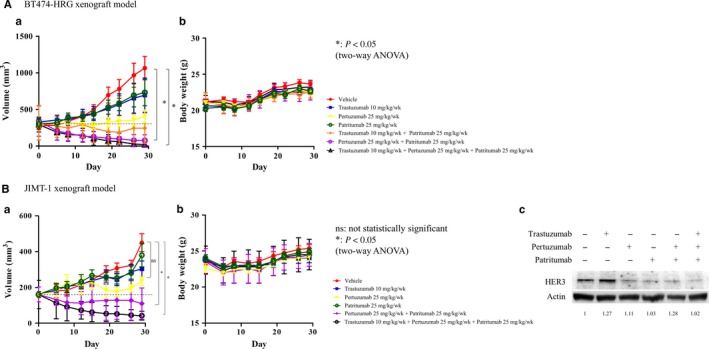
Efficacy of pertuzumab and patritumab with or without trastuzumab on BT474‐HRG and JIMT‐1 tumor xenograft models. A, Vehicle (PBS: 100 μL), trastuzumab (10 mg/kg), pertuzumab (25 mg/kg), patritumab (25 mg/kg), trastuzumab (10 mg/kg) + patritumab (25 mg/kg), pertuzumab (25 mg/kg) + patritumab (25 mg/kg), and trastuzumab (10 mg/kg) + pertuzumab (25 mg/kg) + patritumab (25 mg/kg) were administered via intraperitoneal injection once per week to mice bearing BT474‐HRG cell xenografts. B, Vehicle (PBS: 100 μL), trastuzumab (10 mg/kg), pertuzumab (25 mg/kg), patritumab (25 mg/kg), pertuzumab (25 mg/kg) + patritumab (25 mg/kg), and trastuzumab (10 mg/kg) + pertuzumab (25 mg/kg) + patritumab (25 mg/kg) were administered via intraperitoneal injection once per week to mice bearing JIMT‐1 cell xenografts. Tumor volume (a) and body weights (b) were measured twice per week. Data represent the mean ± standard error. *P < 0.05 (two‐way ANOVA). C, Posttreatment expression of HER2 and HER3 in tumors from xenograft‐bearing mice, as determined by immunoblotting. Cell lysates were analyzed for HER2 and HER3 expression, with β‐actin serving as a loading control.
