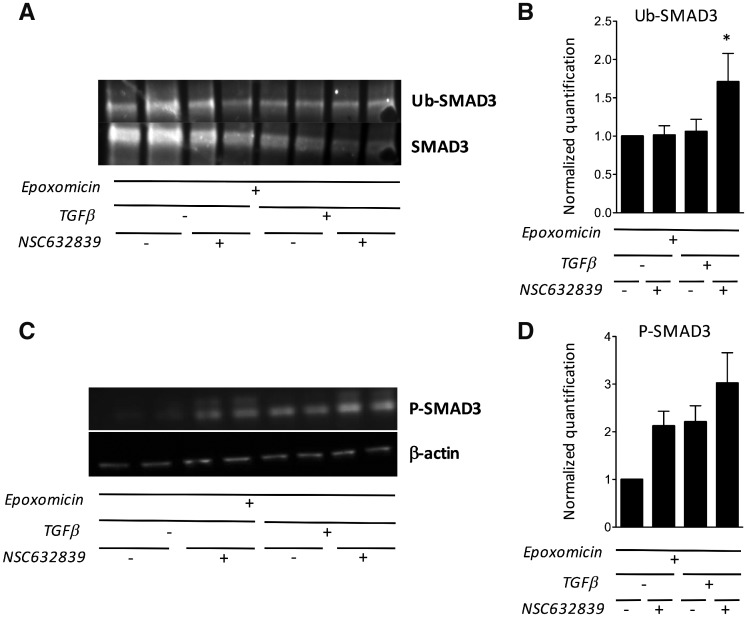
Fig. 5.
NSC632839 induces SMAD3 ubiquitination, and its effects are proteasome-dependent
(A, B) Total SMAD3 was immunoprecipitated from unstimulated or TGF-β-stimulated synovial fibroblasts, cultured in duplicate wells in the presence or the absence of NSC632839. All cells were pre-incubated with epoxomicin, in order to inhibit proteosomal degradation of ubiquitinated SMAD3. The presence of ubiquitinated chains was studied by western blot (Ub-SMAD3) and normalized for total SMAD3 in the immunoprecipitates. (A) Representative experiment. (B) Total SMAD3-normalized quantification of SMAD3 ubiquitination. The results are displayed as the mean (± s.e.m.) from three different experiments. *P < 0.05 using unpaired Student’s t-tests. (C, D) Total and phospho-SMAD3 western blots (C) and β-actin-normalized quantification (D) in unstimulated or TGF-β-stimulated synovial fibroblasts, pre-incubated with epoxomicin, and cultured in duplicate wells in the presence or the absence of NSC632839. Results are displayed as the mean (± s.e.m.) values from three different experiments.