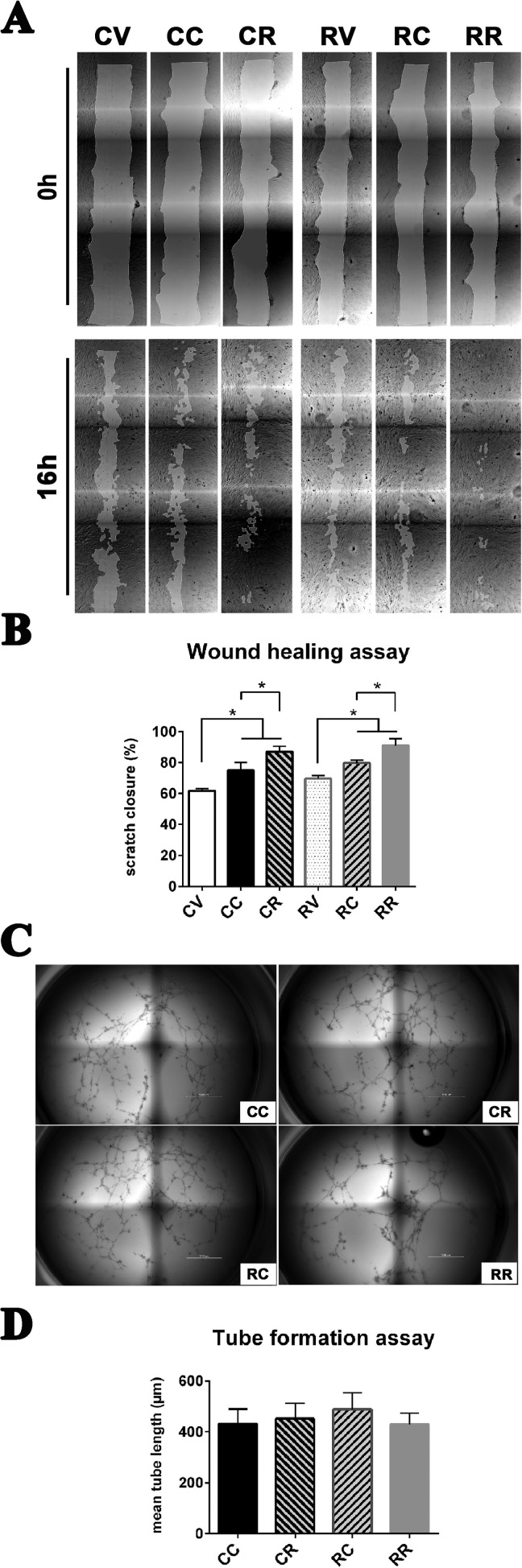
Fig. 2.
Exosomes from Refeed®-supplemented hFM-MSCs enhance their migration ability without modifying vasculogenic properties. (A) Representative images (4× magnification) of the whole wound area in the scratch assay taken at 0 (upper panel) and 16 h (lower panel) after cell monolayer scratching and concurrent supplementation of vehicle or exosomes. Control cells treated Fig. 2 (continued). with vehicle (CV), with exosomes derived from control hFM-MSCs (CC), or with exosomes derived from Refeed®-supplemented hFM-MSCs (CR); Refeed®-supplemented cells treated with vehicle (RV), with exosomes derived from control hFM-MSCs (RC), or with exosomes derived from Refeed®-supplemented hFM-MSCs (RR). (B) The migration rate of control cells supplemented with exosomes derived from Refeed®-supplemented hFM-MSCs (CR and RR) was significantly higher compared with exosomes derived from control hFM-MSCs (CC and RC) or with vehicle (CV and RV). The migration rate is represented as percentage of scratch closure area. *Significant difference between two groups. Each bar represents the mean ± SD (n = 3, one-way analysis of variance with subsequent Tukey’s test, *p < .05). (C) In vitro capillarogenesis assessed in control (C) and Refeed®-supplemented (R) hFM-MSCs exposed to exosomes derived from control (CC or RC) or Refeed®-supplemented (CR or RR) cells. Representative images of total well area in tube formation assays after 12 h. (D) Bar graph showing quantification of tube formation at 12 h in CC, CR, RC, and RR cells. Each bar represents the mean ± SD (n = 6; one-way analysis of variance with subsequent Tukey’s test).