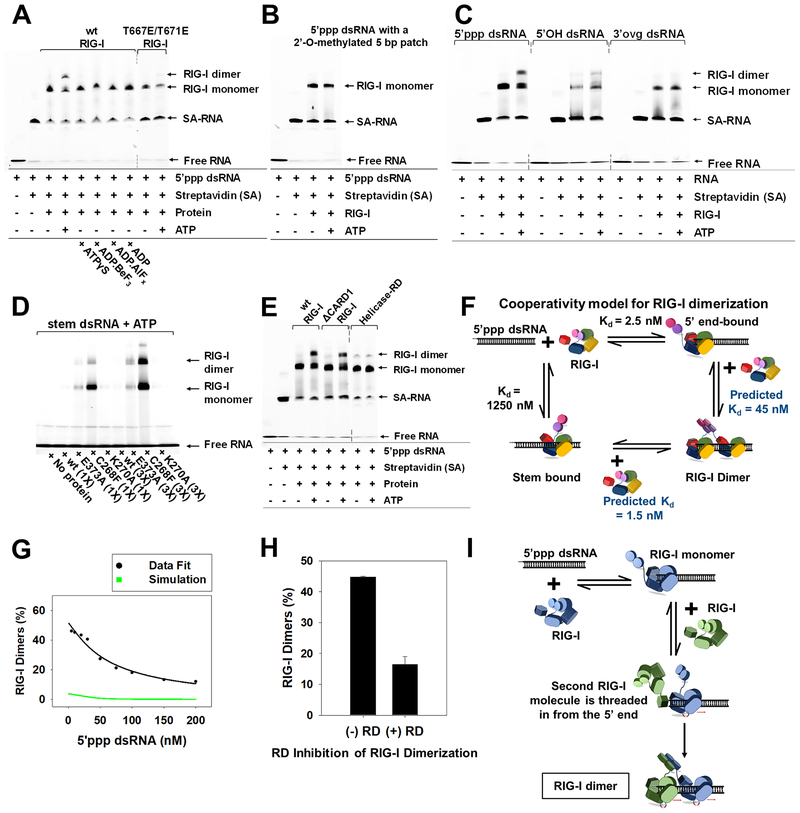
Figure 6. ATP facilitates cooperative RIG-I dimerization on 5’ppp dsRNA by 5’-end threading mechanism.
(A-B) EMSAs of RIG-I or specified mutant (25 nM) with DY547-tagged 5’ppp dsRNA (25 nM) (A) and methylation patch carrying 5’ppp dsRNA (25 nM) (B). (C) EMSAs of DY547 labeled 5’ppp dsRNA, 5’OH dsRNA and 3’ovg dsRNA (100 nM each) with RIG-I (100 nM). (D) EMSAs of Cy3 labeled stem dsRNA (100 nM) with RIG-I and specified mutants (100 and 300 nM each). (E) EMSA of DY547 labeled 5’ppp dsRNA (25 nM) with RIG-I or specified mutant (25 nM) in presence or absence of ATP. (F) Cooperativity model for RIG-I dimerization. (G) RIG-I (50 nM) was titrated with increasing concentrations of 5’ppp dsRNA (5–200 nM) with ATP (2mM) and EMSA was used to determine percent dimers. Green line represents the expected trend for non-cooperative RIG-I dimerization, and the black line shows the best fit to the cooperative dimerization model in E. (H) 5’ppp dsRNA (25 nM) and RIG-I (75 nM) were incubated with and without RD (75 nM) and ATP (2 mM), and percent RIG-I dimers from EMSA are plotted. Error bars are SEM from triplicates. (I) The 5’-end threading model is consistent with results in G. Dotted lines in EMSA gel blots represent deletion of lanes. See also Figure S6.