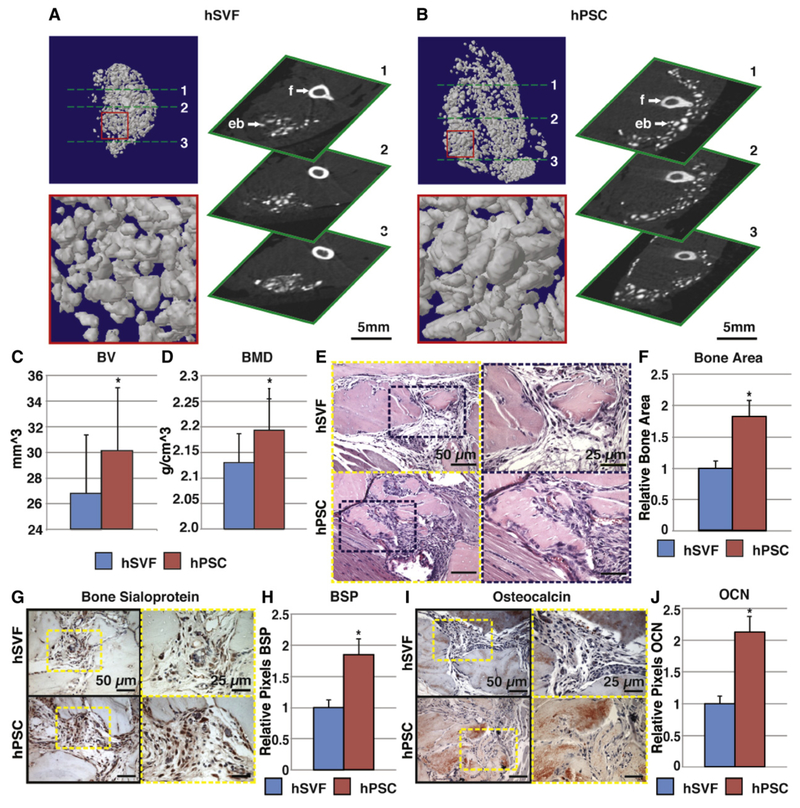
Fig. 4.
hPSC undergo more robust differentiation in comparison to human stromal vascular fraction (hSVF). Equal numbers of viable hSVF cells or hPSC (2.5 × 105) from the same patient samples were implanted intramuscularly in the thigh of a SCID mouse. An osteoinductive demineralized bone matrix (DBX®) putty was used as scaffold. Assessments were performed at 4 weeks post-implantation. (A,B) MicroCT images of hSVF and hPSC-treated samples. Representative 3-dimensional microCT reconstructions at Th90 (left) and corresponding 2-dimensional axial slices (right). (C,D) Analysis of bone volume (BV) and bone mineral density (BMD) among hSVF- and hPSC-treated samples. Th50–120. (E) Representative H&E staining. (F) Representative histomorphometric quantification of bone area per 100× field. (G) Representative bone sialoprotein (BSP) immunohistochemistry and (H) quantification of relative staining per 400× field. (I) Representative osteocalcin (OCN) immunohistochemistry and (J) quantification of relative staining per 400× field. Histomorphometric quantification calculated from N = 6 random microscopical fields. N = 12 implants per cell type, N = 3 patient specimens. *P < 0.05 in comparison to control as assessed by a Student’s t-test. eb: ectopic bone; f: femur. Reproduced with permission from James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, Pang S, Stoker D, Ting K, Peault B, Soo C. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. Jun 2012; 1(6):510–9. PMCID: PMC3659717.