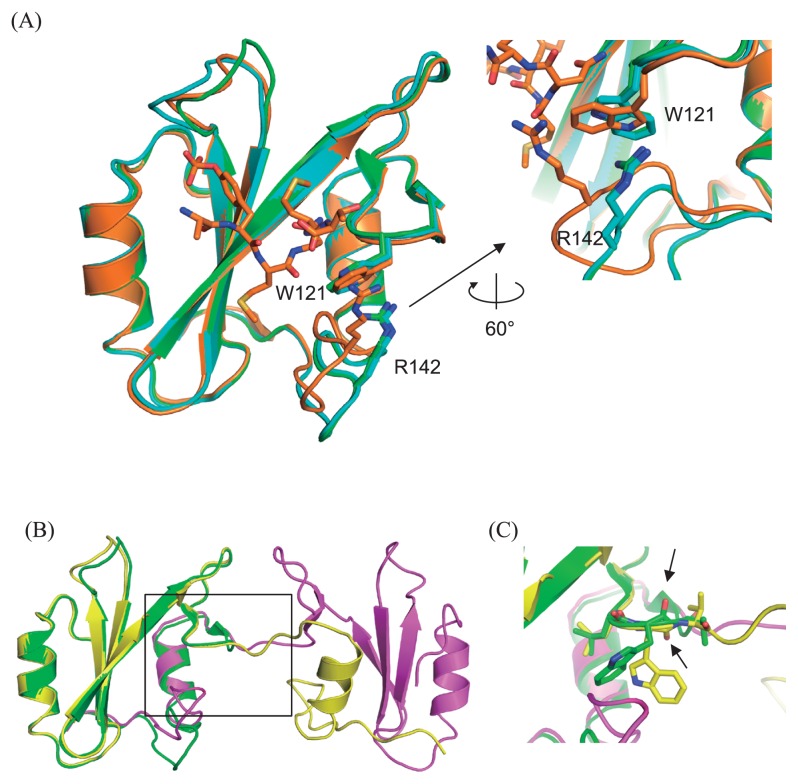
Figure 4.
Overall structures of Grb2 SH2 monomer and dimer. (A) Left: Two monomers in the asymmetric unit (green and cyan) are superimposed on the structure of the complex with CD28 (orange, 3WA4). The root-mean-square-distance (rmsd) are 0.47 (between the monomer shown as green and 3WA4) and 0.54 (between the monomer shown as cyan and 3WA4) Å, respectively. Right: Close-up view of the interaction mode between W121 and R142. (B) Overall structures of the domain swapped dimer (yellow and magenta) superposed on that of one of the monomer (green, same molecule in panel A). (C) Close-up view around the hinge residue W121 (black square in (B)), whose peptide plane flips compared between that of monomer (green) and the domain swapped dimer (yellow), as highlighted by the black allows indicating the oxygen atoms of the main chain carbonyls.