Abstract
We evaluated factors associated with subjective and objective sleepiness at baseline and after 6 months of continuous positive airway pressure (CPAP) therapy in patients with obstructive sleep apnea (OSA).
We analyzed data from the Apnea Positive Pressure Long-term Efficacy Study (APPLES), a prospective 6-month multicenter randomized controlled trial with 1105 subjects with OSA, 558 of who were randomized to active CPAP. Epworth sleepiness scores (ESS) and the mean sleep latency (MSL) on the maintenance of wakefulness test at baseline and after 6 months of CPAP therapy were recorded.
Excessive sleepiness (ESS > 10) was present in 543 (49.1%) participants. Younger age, presence of depression and higher apnea-hypopnea index (AHI) were associated with higher ESS scores and lower MSL. Randomization to the CPAP group was associated with lower odds of sleepiness at 6-months. The prevalence of sleepiness was significantly lower in those using CPAP >4hours /night versus using CPAP ≤4 hours a night. Among those with good CPAP adherence, those with ESS >10 at baseline had significantly higher odds (OR 8.2, P<0.001) of persistent subjective sleepiness.
Lower average nightly CPAP use and presence of sleepiness at baseline were independently associated with excessive subjective and objective sleepiness after 6 months of CPAP therapy.
Keywords: Obstructive sleep apnea, sleep-disordered breathing, Excessive daytime sleepiness, continuous positive airway pressure, CPAP, adherence, compliance, sex, race, age, comorbid, medical disorders, psychiatric disorders
INTRODUCTION
Excessive daytime sleepiness (EDS) is frequently reported in patients with obstructive sleep apnea (OSA). Conversely, OSA has been suggested to be the most common medical disorder that causes EDS. 1 A variety of factors may be associated with EDS in OSA patients. Several studies have assessed the association between the apnea-hypopnea index (AHI) and sleepiness, but while some studies found an association between AHI and EDS, 2–5 others did not.6–8 Obesity, oxygen desaturation index and depressive symptoms have also been associated with EDS in OSA patients. 9–11 However, many of these studies had small sample sizes or did not have objective means of assessment such as the multiple sleep latency test (MSLT) or maintenance of wakefulness test (MWT). Furthermore, while several medical and psychiatric disorders have been associated with poor sleep quality and insomnia,12–14 there are few investigations assessing the impact of comorbid disorders on sleepiness in OSA. Finally, there are few large studies assessing the factors associated with residual sleepiness in patients on CPAP therapy.
The Apnea Positive Pressure Long-term Efficacy Study (APPLES) was a prospective multicenter randomized controlled trial designed to assess the impact of CPAP on several domains of neurocognitive outcomes in adults with OSA. Participants with OSA were randomized to either active CPAP or sham CPAP for 6 months. The subjects completed a number of questionnaires including the Epworth sleepiness scale (ESS) and underwent several tests including the MWT at baseline and 6 months after initiation of CPAP therapy.
In this report, we evaluated the prevalence of subjective and objective sleepiness as well as demographic and polysomnographic variables and medical and psychiatric conditions associated with sleepiness in participants with OSA. We also assessed the factors associated with sleepiness after 6-months of CPAP therapy. Specifically, we aimed to evaluate the impact of different levels of CPAP adherence on prevalence of sleepiness.
METHODS
Study Design
The APPLES protocol has been described in detail elsewhere.15 In brief, participants were recruited from sleep clinics and through public advertisements at 5 sites: Stanford University, Stanford, CA; University of Arizona, Tucson, AZ; Providence St. Mary Medical Center, Walla Walla, WA; St. Luke’s Hospital, Chesterfield, MO; and Brigham and Women’s Hospital, Boston, MA. Those who did not have exclusion criteria during the initial interview and consented to participate in the study underwent a diagnostic polysomnogram (PSG). Those who had an AHI ≥ 10/h without severe oxygen desaturation (i.e., oxygen saturation < 75% for > 10% of the diagnostic sleep study) were randomized to either active or sham CPAP (REMStar Pro, Phillips Respironics, Murrysville, PA) for 6 months. The participants randomized to active CPAP then underwent a titration in the sleep laboratory to determine the optimal therapeutic pressure. A sham titration was performed for those randomized to sham CPAP. Although participants’ adherence was monitored continuously during the study, for this report we calculated adherence at the 6-month time point after initiation of CPAP.
Participants
Of the 1516 participants screened, 1105 were ultimately randomized [268 participants removed from study pre-randomization due to exclusion criteria (e.g., taking exclusionary medication); 143 participants withdrew study “pre-randomization” for any reason (e.g., too busy)],15 with 558 assigned to active CPAP and 547 assigned to sham CPAP. Heated humidification was used in all participants. Staff (e.g., respiratory therapists, sleep technologists, physicians) worked in a systematic manner to troubleshoot nasal congestion and other problems that may have been reported by a participant. Subjects using hypnotics, anxiolytics, sedating antidepressants, anticonvulsants, sedating antihistamines, stimulants, or other medications likely to affect neurocognitive function and/or alertness were excluded from the study. Some subjects were on antidepressants and some on opioids, including that in antitussive medications.
Pre-existing Medical Conditions:
At their baseline visit, all participants underwent a clinical evaluation including a physical examination to obtain a medical history and information pertaining to symptoms of sleep disorders, and presence of other medical and psychiatric conditions such as cardiovascular disease (CVD), gastroesophageal reflux disease (GERD), chronic pain, nasal congestion and depression. A condition was considered present when the participant answered that they currently had it. Furthermore, the Hamilton Rating Scale for Depression (HAMD)16 was administered at baseline and at the 6-month post-CPAP follow-up visit. We considered a score ≥ 8 as indicative of depression being present.
Epworth Sleepiness Scale (ESS)17,18
The ESS is one of the most widely used assessments of subjective daytime sleepiness. It is a self-administered questionnaire where individuals rate their usual chances of dozing off or falling asleep in 8 common situations or activities on a 4-point scale (0 – 3). Hence, the minimum possible score on the scale is 0 (not sleepy at all) and the maximum is 24 (extremely sleepy). The ESS was administered at baseline and at 6 months after starting active CPAP or sham CPAP. Sleepiness was considered clinically significant if the ESS total score was > 10.
Maintenance of Wakefulness Test (MWT)18,19
The MWT test is used to assess objective daytime sleepiness. With the participant sitting in bed with eyes open, it measures sleepiness when the intent is to stay awake. Hence, it provides an objective estimate of the propensity to fall asleep when people would want to stay awake during their normal day. The MWT was performed at baseline and at the 2- and 6-month follow-up visits. For this study, we used the tests performed at the baseline and 6-month follow-up visits. Briefly, after overnight PSG (diagnostic at baseline and with patient’s own CPAP at 6 months), four 20-min trials were conducted at 10:00 AM, 12:00 noon, 2:00 PM, and 4:00 PM. A trial was ended after 20 minutes or after 3 consecutive 30-second epochs of any stage of sleep, whichever occurred first. Once a trial was terminated, the room lights were turned on and the subject was asked to open his or her eyes, stay seated in bed, and remain awake until the 20 min of the trial had elapsed. The mean sleep latency (MSL) of all 4 trials was used for these analyses. Interrater reliability assessments were conducted for PSGs and MWTs blindly scored by the Data Coordinating Center PSG Technologists, and the Data Coordinating Center was responsible for further quality assurance/quality control procedures for these data.15
CPAP Adherence
Adherence to active CPAP or sham CPAP was measured objectively using Encore Pro SmartCards (Phillips Respironics, Inc., Murrysville, PA) that were exchanged at the clinic twice monthly. For this report, at the 6-month period after start of CPAP, the mean hours of daily use were analyzed for the preceding 2-month period to assess adherence during that time interval. The Smart Card is a memory card that records data regarding the duration of each CPAP therapy use and provides reliable objective evidence of CPAP use.
Statistical Analyses
To assess the factors associated with sleepiness at baseline, we included all 1105 participants. For the 6-month analyses, we focused only on those randomized to active CPAP therapy with relevant data available. For assessment of residual sleepiness in those with good adherence, analyses were limited to those participants who were using CPAP >4 hours a night. For continuous variables, unadjusted comparisons between groups were made using Student’s unpaired t-test. Data were expressed as mean ± SD. Differences in proportions were assessed using the χ2 test. Logistic regression was used to assess odds ratios (with 95% confidence intervals) for sleepiness (ESS >10) at baseline and at 6 months after CPAP initiation, with predictors of interest including demographic and polysomnographic characteristics and comorbid conditions. A multiple regression model was used to examine the association between the ESS scores and MSL values as dependent variables, with demographic and polysomnographic characteristics and comorbid conditions at baseline and at 6 months. The statistical significance level was set at P<0.05 (2-tailed) for all tests. Statistical analyses were conducted using SPSS v 20.0 for Windows (SSPS Inc, Chicago, IL).
RESULTS
Sleepiness at baseline
A total of 1105 participants were assessed, including 382 women and 723 men, of whom 611 (55%) had severe OSA defined as AHI >30. The baseline demographics for all participants and subsequently randomized participants stratified by gender are provided in an earlier publication.20 Briefly, the mean age of the participants was 51.5 (12.2), mean BMI was 32.2 (7.1) mean AHI was 38.2 (26.7) and the mean Oxygen Desaturation Index was 25.5 (25.1). The mean baseline ESS of the participants was 10.4±4.4 (minimum 0, maximum 22). In bivariate analyses, women were significantly sleepier (ESS 10.9±4.5 vs. 10.2±4.3, P=0.006) than men. There was a trend for severe sleep apnea to be sleepier than those without severe sleep apnea (ESS 10.7±4.5 vs. 10.2±4.3, P=0.07). The ESS scores correlated directly with BMI, several polysomnographic variables including AHI, and HAMD score, and inversely with age (Table 1). Of the 1105 participants, 543 (49.1%) had excessive sleepiness (ESS > 10). As shown in Table 2, the ESS total scores as well as proportion of participants who were sleepy (ESS > 10) were higher in those with depression and chronic pain. Except for a trend towards greater sleepiness in those with GERD, no differences were observed in a variety of other medical and psychiatric conditions. Of all subjects, 196 were on antidepressants and 73 were on opiates. The use of antidepressants (P=0.31) or opiates (P=0.15) was not significantly associated with greater sleepiness.
Table 1:
Bivariate correlation between clinical and PSG variables and the ESS total score at baseline.
| Variable | Pearson Correlation | P |
|---|---|---|
| Age | −0.07 | 0.02 |
| BMI | 0.10 | 0.001 |
| AHI | 0.08 | 0.01 |
| Desaturation Index | 0.08 | 0.01 |
| Sleep Efficiency | 0.09 | 0.02 |
| HAMD total score | 0.13 | <0.001 |
PSG: Polysomnographic
ESS: Epworth Sleepiness Scale
BMI: Body Mass Index
AHI: Apnea-Hypopnea Index
HAMD: Hamilton Rating Scale for Depression
Table 2:
The mean ESS total score and proportion of sleepy participants in various medical and psychiatric disorders.
| Condition (n with condition/ n without condition) | Mean ESS | P value | % of participants with ESS> 10 | P value | ||
|---|---|---|---|---|---|---|
| Condition present | Condition absent | Condition present | Condition absent | |||
| CVD (399/706) | 10.2 ± 4.4 | 10.6 ± 4.4 | 0.26 | 47.1 | 50.3 | 0.32 |
| Asthma (76/1022) | 10.6 ± 4.7 | 10.4 ± 4.4 | 0.81 | 51.3 | 48.9 | 0.39 |
| GERD (316/782) | 10.8 ± 4.3 | 10.3 ± 4.4 | 0.06 | 52.2 | 47.8 | 0.20 |
| Nocturia (322/776) | 10.7 ± 4.4 | 10.3 ± 4.4 | 0.16 | 52.8 | 47.6 | 0.13 |
| Chronic Pain (173/925) | 11.1 ± 4.5 | 10.3 ± 4.4 | 0.03* | 53.2 | 48.3 | 0.14 |
| DM (74/1024) | 10.6 ± 4.7 | 10.4 ± 4.4 | 0.41 | 76.9 | 68.5 | 0.51 |
| Self-reported Depression (163/935) | 11.0 ± 4.2 | 10.3 ± 4.4 | 0.08* | 52.8 | 48.4 | 0.35 |
| Depression (HAMD Total Score ≥8, 204/894) | 11.4 ± 4.7 | 10.2 ± 4.3 | <0.001* | 57.8 | 47.0 | 0.005* |
| Anxiety (76/1021) | 11.0 ± 4.0 | 10.4 ± 4.4 | 0.23 | 52.6 | 48.8 | 0.48 |
| Claustrophobia (88/1008) | 10.9 ± 4.7 | 10.4 ± 4.4 | 0.24 | 51.1 | 48.8 | 0.32 |
ESS: Epworth Sleepiness Scale
CVD: Cardiovascular Disease
GERD: Gastroesophageal Reflux Disease
DM: Diabetes Mellitus
HAMD: Hamilton Rating Scale for Depression
A linear regression model revealed younger age, higher AHI and elevated HAMD scores to be independently associated with higher ESS total scores (Table 3a). Logistic regression showed higher odds of sleepiness in those with depression (HAMD scores ≥8, OR=1.4, P=0.03) and lower odds with increasing age (Table 3b).
Table 3a:
Linear regression analysis with ESS total score as the dependent variable
| Variable | Beta | Std. Error | Standardized Coefficient (Beta) | t | P |
|---|---|---|---|---|---|
| Age | −0.025 | 0.011 | −0.068 | −2.211 | 0.027* |
| Gender (Female) | 0.530 | 0.292 | 0.057 | 1.812 | 0.070 |
| BMI | 0.021 | 0.021 | 0.033 | 0.984 | 0.325 |
| AHI | 0.012 | 0.006 | 0.066 | 2.009 | 0.045* |
| HAMD Total Score ≥8 | 0.118 | 0.033 | 0.109 | 3.556 | <0.001* |
| Chronic Pain | 0.609 | 0.373 | 0.050 | 1.631 | 0.103 |
| GERD | 0.364 | 0.297 | 0.037 | 1.227 | 0.220 |
ESS: Epworth Sleepiness Scale
BMI: Body Mass Index
AHI: Apnea-Hypopnea Index
HAMD: Hamilton Rating Scale for Depression
GERD: Gastroesophageal Reflux Disease
Table 3b:
Odds of ESS > 10 at baseline
| Variable | ESS>10 (n=543) | ESS<10 (n=562) | Wald | Odds Ratio | P |
|---|---|---|---|---|---|
| Age (years) | 50.4 ± 11.9 | 52.7 ± 12.4 | 9.318 | 0.984 | 0.002* |
| Gender (Female), n (%) | 206 (37.9%) | 176 (31.3%) | 3.493 | 1.290 | 0.062 |
| BMI (kg/m2) | 32.7 ± 6.9 | 31.7 ± 7.3 | 0.108 | 1.003 | 0.742 |
| Baseline AHI >30, n (%) | 312 (57.5%) | 299 (53.2%) | 2.974 | 1.005 | 0.085 |
| HAMD Total Score ≥8, n (%) | 118 (21.9%) | 86 (15.4%) | 4.732 | 1.423 | 0.030* |
| Chronic Pain, n (%) | 92 (17.1%) | 81 (14.5%) | 0.624 | 1.148 | 0.429 |
| GERD, n (%) | 165 (30.6%) | 151 (27.0%) | 1.010 | 1.149 | 0.315 |
ESS: Epworth Sleepiness Scale
BMI: Body Mass Index
AHI: Apnea-Hypopnea Index
HAMD: Hamilton Rating Scale for Depression
GERD: Gastroesophageal Reflux Disease
The MWT MSL at baseline (MSL-B, n=1086) was 17.0 minutes (minimum 1.9 minutes, maximum 20 minutes). An MSL-B equal to 20 minutes was seen in 521 participants (48%) and an MSL-B <20 minutes in 565 participants (52%). For these analyses, a MSL <20 minutes was considered abnormal. Notably, we repeated analyses using MSL ≤17 minutes as abnormal, which was seen in 37.5% (n=407) of all participants at baseline. The results obtained using this cutoff to define sleepiness were generally similar to those obtained using MSL <20 minutes as abnormal.
As expected, MSL-B was significantly lower in those with ESS >10 than those with ESS ≤10 (16.3±4.3 minutes vs. 17.7±3.5 minutes, P<0.001). Of those with ESS >10, 61.2% had a MSL-B <20 minutes compared to only 43.2% in those with ESS ≤10 (P<0.001). Logistic regression showed that younger age, presence of depression and AHI >30 were associated with higher odds of sleepiness (MSL-B <20 minutes) (Table 3c).
Table 3c:
Odds of objective sleepiness (MWT < 20 minutes) at baseline
| Variable | MWT < 20 (n=521) | MWT = 20 (n=565) | Odds Ratio | P |
|---|---|---|---|---|
| Age (years) | 49.6 ± 12.6 | 53.8 ± 11.3 | 0.972 | 0.000* |
| Gender (Female), n (%) | 196 (34.7%) | 178 (34.2%) | 0.977 | 0.868 |
| BMI (kg/m2) | 33.1 ± 7.4 | 31.2 ± 6.6 | 1.018 | 0.064 |
| Baseline AHI >30, n (%) | 354 (62.7%) | 249 (47.8%) | 1.858 | <0.001* |
| HAMD Total Score ≥8, n (%) | 123 (21.9%) | 79 (15.2%) | 1.500 | 0.016* |
| Chronic Pain, n (%) | 91 (16.1%) | 81 (15.5%) | 1.081 | 0.664 |
| GERD, n (%) | 161 (28.5%) | 149 (28.6%) | 0.921 | 0.563 |
MWT: Maintenance of Wakefulness Test
BMI: Body Mass Index
AHI: Apnea-Hypopnea Index
HAMD: Hamilton Rating Scale for Depression
GERD: Gastroesophageal Reflux Disease
Sleepiness at 6 months after CPAP initiation
The mean age of the participants randomized to CPAP was 52.2 ± 12.1 years, mean BMI was 32.3 ± 7.4, mean AHI was 39.5 ± 24.9 and the mean Oxygen Desaturation Index was 26.3 ± 24.1. At 6-month period, the mean AHI in this group was 6.1 ± 8.3 and the mean Oxygen Desaturation Index was 4.8 ±7.3. In contrast, the mean AHI in the sham group at 6 months was 29.8 ± 25.1 and the mean Oxygen Desaturation Index was 24.8 ±24.9. The ESS was significantly lower in the CPAP group compared to the sham group at 2-month (7.9 vs. 8.8, P=0.003), 4- month (7.0 vs. 8.2, P<0.001) and 6- month (7.3 vs. 8.4, P=0.003) periods after initiation of therapy despite similar ESS at baseline (10.5 vs. 10.5, P=0.99).
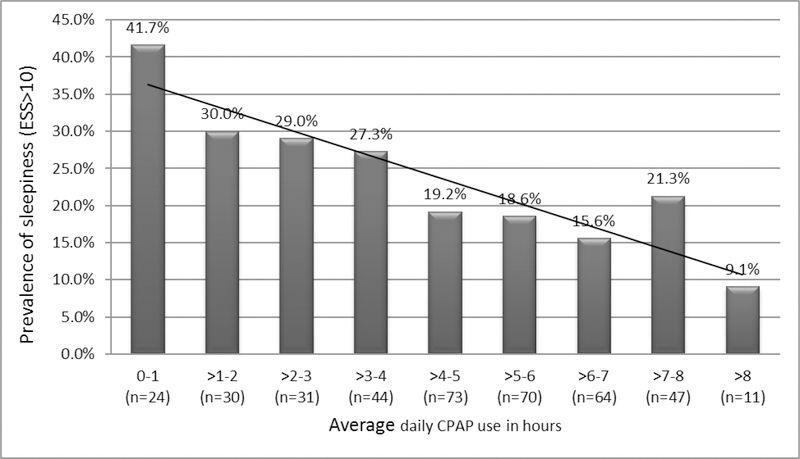
At 6 months, 88 (22.3%) of the 394 participants initiated on CPAP had excessive sleepiness defined as ESS >10. The mean nightly adherence at 6 months in those randomized to active CPAP was 4.70 ± 2.04 hours and 67.3% of the participants were using CPAP > 4 h per night. Further details of adherence in the study participants are provided in an earlier publication.21 The prevalence of sleepiness generally declined progressively with increasing CPAP use (Figure 1). The prevalence of sleepiness was higher in those using CPAP ≤4 hours/night versus those using CPAP >4hours a night (31% vs. 18%, P=0.003). Fewer hours of CPAP use and higher ESS total scores at baseline were independently associated with higher 6-month ESS total scores in a linear regression model (Table 4a). A logistic regression model (Table 4b) showed lower odds of sleepiness in those using CPAP >4 hours a night (OR=0.42, P=0.001), and higher odds in those who were sleepy at baseline (OR=5.1, P<0.001). Women had lower odds of sleepiness than men, the presence of chronic pain was associated with higher odds and the presence of depression was associated with a trend towards higher odds of sleepiness (Table 4b). The use of antidepressants (n=100, P=0.4) or opiates (n=38, P=0.08) was not significantly associated with ESS >10.
Figure 1:
The prevalence of sleepiness in all participants with different mean daily CPAP use.
CPAP: Continuous Positive Airway Pressure
ESS: Epworth Sleepiness Scale
Table 4a:
Linear regression analysis with ESS total score at 6 months as the dependent variable
| Variable | Beta | Std. Error | Standardized Coefficient (Beta) | t | P |
|---|---|---|---|---|---|
| Age | 0.020 | 0.015 | 0.058 | 1.328 | 0.185 |
| Gender (Female) | −0.548 | 0.374 | −0.062 | −1.466 | 0.143 |
| BMI | −0.031 | 0.025 | −0.055 | −1.210 | 0.227 |
| HAMD Total Score | 0.023 | 0.040 | 0.023 | 0.566 | 0.571 |
| Chronic Pain | 0.766 | 0.462 | 0.069 | 1.660 | 0.098 |
| GERD | 0.045 | 0.380 | 0.005 | 0.119 | 0.906 |
| Baseline ESS | 0.495 | 0.039 | 0.528 | 12.679 | <0.001* |
| AHI Baseline | −0.007 | 0.008 | −0.038 | −0.841 | 0.401 |
| AHI at 6 Months | −0.025 | 0.022 | −0.049 | −1.144 | 0.253 |
| Mean Hours of CPAP Adherence | −0.240 | 0.067 | −0.151 | −3.579 | <0.001* |
ESS: Epworth Sleepiness Scale
BMI: Body Mass Index
HAMD: Hamilton Rating Scale for Depression
GERD: Gastroesophageal Reflux Disease
AHI: Apnea-Hypopnea Index
CPAP: Continuous Positive Airway Pressure
Table 4b:
Odds of ESS >10 at 6 months
| Variable | ESS>10 (n=88) | ESS<10 (n=306) | Odds Ratio | P |
|---|---|---|---|---|
| Age (years) | 51.2 ± 12.5 | 53.0 ± 12.0 | 1.001 | 0.920 |
| Gender (Female), n (%) | 29 (33.0%) | 106 (34.6%) | 0.582 | 0.049* |
| BMI (kg/m2) | 31.6 ± 6.8 | 32.2 ± 7.3 | 0.982 | 0.356 |
| HAMD Total Score ≥8, n (%) | 19 (21.6%) | 51 (16.7%) | 1.766 | 0.059 |
| Chronic Pain, n (%) | 23 (26.1%) | 45 (14.7%) | 2.289 | 0.008* |
| GERD, n (%) | 29 (33.0%) | 86 (28.1%) | 0.979 | 0.939 |
| Baseline ESS >10, n (%) | 65 (73.9%) | 127 (41.5%) | 5.143 | <0.001* |
| Baseline AHI >30, n (%) | 48 (54.5%) | 171 (55.9%) | 1.106 | 0.715 |
| AHI >5 at 6 Months, n (%) | 27 (30.7%) | 111 (36.3%) | 0.754 | 0.291 |
| CPAP Use >4 Hours/Night, n (%) | 48 (54.5%) | 217 (70.9%) | 0.425 | 0.001* |
ESS: Epworth Sleepiness Scale
BMI: Body Mass Index
HAMD: Hamilton Rating Scale for Depression
GERD: Gastroesophageal Reflux Disease
AHI: Apnea-Hypopnea Index
CPAP: Continuous Positive Airway Pressure
The MWT and CPAP adherence data were available for 380 participants randomized to CPAP, 6-months after initiation of therapy. The mean sleep latency on MWT at 6–months (MSL-6m) was 18 minutes. MSL-6m was significantly higher in those using CPAP >4hours a night compared to those using CPAP 4 hours or less (18.5±2.8 vs. 17.5±3.8 minutes, P=0.01). MSL-6m was <20 minutes in 147 participants (38.7%) and ≤17 minutes in 90 participants (23.7%). Logistic regression revealed significantly lower odds of excessive sleepiness, defined as MSL-6m <20 minutes (MSL-6m <20), in those using CPAP >4 hours a night (OR=0.55, P=0.008). Those with MSL-B <20 minutes also had significantly higher odds of MSL-6m <20 (OR=6.7, P<0.001) (Table 4c). Using a cutoff of 17 minutes for MSL-6m to define sleepiness yielded similar results.
Table 4c:
Odds of objective sleepiness (MSL-6m <20 minutes) at 6 months
| Variable | MSL-6m < 20 (n=147) | MSL-6m = 20 (n=233) | Odds Ratio | P |
|---|---|---|---|---|
| Age (years) | 53.8 ± 12.4 | 53.8 ± 11.4 | .994 | .521 |
| Gender (Female), n (%) | 46 (31.3%) | 84 (36.1%) | .678 | .109 |
| BMI (kg/m2) | 32.2 ± 6.7 | 31.9 ± 7.2 | 1.004 | .794 |
| HAMD Total Score ≥8, n (%) | 30 (20.4%) | 39 (16.7%) | 1.168 | .587 |
| Chronic Pain, n (%) | 24 (16.3%) | 43 (18.5%) | .827 | .524 |
| GERD, n (%) | 49 (33.3%) | 65 (27.9%) | 1.249 | .364 |
| Baseline MWT<20, n (%) | 156 (67.0%) | 33 (22.4%) | 6.711 | .000* |
| Baseline AHI >30, n (%) | 80 (54.4%) | 128 (54.9%) | .811 | .398 |
| AHI >5 at 6 Months, n (%) | 51 (34.7%) | 84 (36.1%) | 1.020 | .933 |
| CPAP Use >4 Hours/Night, n (%) | 85 (57.8%) | 169 (72.5%) | .547 | .008* |
MSL-6M: Mean Sleep Latency- 6 Months
BMI: Body Mass Index
HAMD: Hamilton Rating Scale for Depression
GERD: Gastroesophageal Reflux Disease
MWT: Maintenance of Wakefulness Test
AHI: Apnea-Hypopnea Index
CPAP: Continuous Positive Airway Pressure
Sleepiness at 6 months in participants with good adherence to CPAP
The prevalence of ESS >10 was 18.1% (48/265) among those using CPAP >4 hours a night.
The presence of excessive sleepiness at 6 months in these patients with good CPAP adherence was higher in those with baseline excessive sleepiness, whether assessed by ESS >10 (OR 8.2, P<0.001) or MSL-B <20 minutes (OR=9.6, P<0.001). No other variables assessed, including age, BMI, AHI on the sleep study done at 6-months, presence of depression, chronic pain or GERD, were significantly associated with odds of sleepiness at 6 months in this group of patients.
DISCUSSION
The current study found that excessive sleepiness, as measured by ESS total score >10 or a MSL <20 minutes on MWT, was present in half the patients with sleep apnea, and was associated with presence of depression, more severe sleep apnea and younger age. The prevalence of excessive sleepiness 6 months after initiating CPAP was greatly reduced to 22.3%, and was inversely associated with mean hours of CPAP use. Presence of excessive sleepiness at baseline was a strong determinant of excessive sleepiness after 6 months of therapy, even in those who had good adherence to CPAP.
We found that younger age, higher AHI and presence of depression are associated with increased sleepiness in patients with sleep apnea. The past literature assessing association between AHI and EDS has shown conflicting results, with some studies 2–5 but not others6–8 showing an association. The studies that have not shown this association probably reflect a referral bias, since sleepy patients are more likely to be referred for sleep evaluation, thereby obscuring the relation of AHI to sleepiness that is seen in population-based studies.
The association between depression and increased sleepiness confirms findings of prior reports 10,22,23. Depression is a common finding in patients with OSA. We found an 18.6% prevalence of depression, defined as HAMD score ≥8. This is similar to a 20.8% prevalence of depression found in patients with untreated OSA in a recent study. 22 Similar to that study, we found an association between depression and ESS. This strongly argues for making appropriate inquiries for the presence of depression in those with SDB, especially in presence of sleepiness.
The ESS scores and prevalence of sleepiness decreased with increasing age, as has been reported in some prior studies. 23,24 This may be related to less potent sleep homeostasis mechanisms with increasing age, including morphological and neurological changes in suprachiasmatic nucleus and a reduction in melatonin concentration with aging.25 These findings suggest that complaints of sleepiness in the elderly would represent a less common response, and should not be considered a normal part of the physiologic aging process.
Lower average nightly CPAP use and presence of sleepiness at baseline were the primary determinants of residual sleepiness identified in this study. The effect of good adherence on reducing sleepiness confirms the benefit of this therapy demonstrated in the past studies.26–28 We found, however, that after 6 months of CPAP therapy, 22.3% of the participants on CPAP (18.1% of those using CPAP >4 hours a night) still had sleepiness. This prevalence is close to that noted in recent studies,27,28 and is similar to the prevalence of excessive sleepiness in the general population among those without sleep apnea.4 Thus, while increasing CPAP adherence beyond 4 hours per night might yield some further reduction in daytime sleepiness, much of the residual sleepiness in these patents may be caused by factors other than OSA, indicating the importance of evaluating patients with residual sleepiness on CPAP for alternative causes of sleepiness. Oxidative injury to sleep-wake brain regions from long-term intermittent hypoxia has been suggested as a reason for residual sleepiness following treatment of OSA.29 However, reasons for sleepiness, such as chronic pain or other conditions contributing to repeated wakefulness at night, sedative medications, depression, poor sleep hygiene or insufficient habitual sleep duration should be sought and modified before attributing residual sleepiness to a failure of CPAP therapy. In view of the finding that those who were sleepy at baseline had significantly higher odds (OR 8.2, P<0.001) of sleepiness despite good CPAP adherence, it is imperative that these patients be closely followed to ensure improvement in sleepiness.
The study has several limitations. First, we used the ESS for assessment of sleepiness in patients with OSA. The ESS, while a commonly used measure of sleepiness, has only a limited correlation with objective sleepiness measurements.30 However, we also used the MWT, an objective measurement of ability to resist sleep onset, and the results were similar to those obtained using subjective measurements from the ESS. Second, while we used MWT-SL to objectively assess ability to maintain wakefulness, the optimal MWT cutoff values in those with OSA have not been defined. To overcome this limitation, we used different cutoff values to decide whether the patients had decreased wakefulness. Notably, we obtained similar predictors of sleepiness while using these different cutoffs, and the results were consistent with those obtained using ESS. Third, the information about medical and psychiatric conditions was self-reported in most cases and obtained only at baseline. Hence, whether the conditions were present at the 6-month evaluation period, and may have influenced sleepiness, is not clear. Furthermore, apart from depression, for which we had HAMD scale values, the severity of other conditions was not assessed. Fourth, residual confounding by several factors including habitual sleep duration, disorders not documented in the study, medications, and genetic and socioeconomic factors cannot be excluded.31 Finally, the standard MWT administration consists of 40-minute nap opportunities, while the naps in this trial were only 20 minutes. However, the resultant ceiling effect is expected to reduce power to detect effects of treatment and makes the differences with therapy even more compelling.
In conclusion, this study shows that depression, younger age and higher AHI, are associated with sleepiness in people with OSA. CPAP use is associated with reduced odds of sleepiness at 6 months. Lower average nightly CPAP use and presence of sleepiness at baseline are the primary determinants of persistent sleepiness in those using CPAP therapy, even among CPAP-adherent patients. Specific strategies targeting these patients, including stressing the role of CPAP in decreasing sleepiness, frequent follow-up clinic visits to evaluate residual sleepiness and promote adherence,21,32 as well as thorough evaluation for other etiologies of sleepiness should be strongly considered. An appreciable number of OSA patients will continue to have sleepiness despite using CPAP. Other causes of sleepiness and other measures including addition of wake-promoting medications should be considered in these patients.
ACKNOWLEDGMENTS
The Apnea Positive Pressure Long-term Efficacy Study (APPLES) study was funded by contract 5UO1-HL-068060 from the National Heart, Lung and Blood Institute. The APPLES pilot studies were supported by grants from the American Academy of Sleep Medicine and the Sleep Medicine Education and Research Foundation to Stanford University and by the National Institute of Neurological Disorders and Stroke (N44-NS-002394) to SAM Technology. In addition, APPLES investigators gratefully recognize the vital input and support of Dr. Sylvan Green, who died before the results of this trial were analyzed, but was instrumental in its design and conduct.
Administrative Core: Clete A. Kushida, MD, PhD; Deborah A. Nichols, MS; Eileen B. Leary, BA, RPSGT; Pamela R. Hyde, MA; Tyson H. Holmes, PhD; Daniel A. Bloch, PhD; William C. Dement, MD, PhD
Data Coordinating Center: Daniel A. Bloch, PhD; Tyson H. Holmes, PhD; Deborah A. Nichols, MS; Rik Jadrnicek, Microflow, Ric Miller, Microflow Usman Aijaz, MS; Aamir Farooq, PhD; Darryl Thomander, PhD; Chia-Yu Cardell, RPSGT; Emily Kees, Michael E. Sorel, MPH; Oscar Carrillo, RPSGT; Tami Crabtree, MS; Booil Jo, PhD; Ray Balise, PhD; Tracy Kuo, PhD
Clinical Coordinating Center: Clete A. Kushida, MD, PhD, William C. Dement, MD, PhD, Pamela R. Hyde, MA, Rhonda M. Wong, BA, Pete Silva, Max Hirshkowitz, PhD, Alan Gevins, DSc, Gary Kay, PhD, Linda K. McEvoy, PhD, Cynthia S. Chan, BS, Sylvan Green, MD
Clinical Centers
Stanford University: Christian Guilleminault, MD; Eileen B. Leary, BA, RPSGT; David Claman, MD; Stephen Brooks, MD; Julianne Blythe, PA-C, RPSGT; Jennifer Blair, BA; Pam Simi, Ronelle Broussard, BA; Emily Greenberg, MPH; Bethany Franklin, MS; Amirah Khouzam, MA; Sanjana Behari Black, BS, RPSGT; Viola Arias, RPSGT; Romelyn Delos Santos, BS; Tara Tanaka, PhD
University of Arizona: Stuart F. Quan, MD; James L. Goodwin, PhD; Wei Shen, MD; Phillip Eichling, MD; Rohit Budhiraja, MD; Charles Wynstra, MBA; Cathy Ward, Colleen Dunn, BS; Terry Smith, BS; Dane Holderman, Michael Robinson, BS; Osmara Molina, BS; Aaron Ostrovsky, Jesus Wences, Sean Priefert, Julia Rogers, BS; Megan Ruiter, BS; Leslie Crosby, BS, RN
St. Mary Medical Center: Richard D. Simon Jr., MD; Kevin Hurlburt, RPSGT; Michael Bernstein, MD; Timothy Davidson, MD; Jeannine Orock-Takele, RPSGT; Shelly Rubin, MA; Phillip Smith, RPSGT; Erica Roth, RPSGT; Julie Flaa, RPSGT; Jennifer Blair, BA; Jennifer Schwartz, BA; Anna Simon, BA; Amber Randall, BA
St. Luke’s Hospital: James K. Walsh, PhD, Paula K. Schweitzer, PhD, Anup Katyal, MD, Rhody Eisenstein, MD, Stephen Feren, MD, Nancy Cline, Dena Robertson, RN, Sheri Compton, RN, Susan Greene, Kara Griffin, MS, Janine Hall, PhD
Brigham and Women’s Hospital: Daniel J. Gottlieb, MD, MPH, David P. White, MD, Denise Clarke, BSc, RPSGT, Kevin Moore, BA, Grace Brown, BA, Paige Hardy, MS, Kerry Eudy, PhD, Lawrence Epstein, MD, Sanjay Patel, MD
Sleep HealthCenters for the use of their clinical facilities to conduct this research
Consultant Teams
Methodology Team: Daniel A. Bloch, PhD, Sylvan Green, MD, Tyson H. Holmes, PhD, Maurice M. Ohayon, MD, DSc, David White, MD, Terry Young, PhD
Sleep-Disordered Breathing Protocol Team: Christian Guilleminault, MD, Stuart Quan, MD, David White, MD
EEG/Neurocognitive Function Team: Jed Black, MD, Alan Gevins, DSc, Max Hirshkowitz, PhD, Gary Kay, PhD, Tracy Kuo, PhD
Mood and Sleepiness Assessment Team: Ruth Benca, MD, PhD, William C. Dement, MD, PhD, Karl Doghramji, MD, Tracy Kuo, PhD, James K. Walsh, PhD
Quality of Life Assessment Team: W. Ward Flemons, MD, Robert M. Kaplan, PhD
APPLES Secondary Analysis-Neurocognitive (ASA-NC) Team: Dean Beebe, PhD, Robert Heaton, PhD, Joel Kramer, PsyD, Ronald Lazar, PhD, David Loewenstein, PhD, Frederick Schmitt, PhD
National Heart, Lung, and Blood Institute (NHLBI)
Michael J. Twery, PhD, Gail G. Weinmann, MD, Colin O. Wu, PhD
Data and Safety Monitoring Board (DSMB)
Seven-year term: Richard J. Martin, MD (Chair), David F. Dinges, PhD, Charles F. Emery, PhD, Susan M. Harding MD, John M. Lachin, ScD, Phyllis C. Zee, MD, PhD
Other term: Xihong Lin, PhD (2 y), Thomas H. Murray, PhD (1 y).
Abbreviations:
- OSA
Obstructive sleep apnea
- CPAP
Continuous positive airway pressure
- EDS
Excessive daytime sleepiness
- BMI
Body Mass Index
- AHI
Apnea-Hypopnea Index
- CVD
Cardiovascular Disease
- GERD
Gastroesophageal Reflux Disease
- DM
Diabetes Mellitus
- HAMD
Hamilton Rating Scale for Depression
- ESS
Epworth Sleepiness Scale
- MWT
Maintenance of Wakefulness Test
- MSL
Mean sleep latency
- MSL-B
Mean sleep latency on the Maintenance of Wakefulness Test at baseline
- MSL-6m
Mean sleep latency on the Maintenance of Wakefulness Test at 6-months after CPAP initiation
Footnotes
None of the authors claim any financial support or conflicts of interest related to this manuscript. The manuscript does not contain any off-label or investigational use.
REFERENCES
- 1.Strohl KP, Brown DB, Collop N, George C, Grunstein R, Han F, Kline L, Malhotra A, Pack A, Phillips B and others. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med 2013;187(11):1259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto FJ. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep 2005;28(4):472–7. [DOI] [PubMed] [Google Scholar]
- 3.Roure N, Gomez S, Mediano O, Duran J, Peña MeL, Capote F, Teran J, Masa JF, Alonso ML, Corral Jand others. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med 2008;9(7):727–31. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Whitney CW, Bonekat WH, Iber C, James GD, Lebowitz M, Nieto FJ, Rosenberg CE. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med 1999;159(2):502–7. [DOI] [PubMed] [Google Scholar]
- 5.Bjorvatn B, Lehmann S, Gulati S, Aurlien H, Pallesen S, Saxvig IW. Prevalence of excessive sleepiness is higher whereas insomnia is lower with greater severity of obstructive sleep apnea. Sleep Breath 2015. [DOI] [PubMed] [Google Scholar]
- 6.Dixon JB, Dixon ME, Anderson ML, Schachter L, O’brien PE. Daytime sleepiness in the obese: not as simple as obstructive sleep apnea. Obesity (Silver Spring) 2007;15(10):2504–11. [DOI] [PubMed] [Google Scholar]
- 7.Mediano O, Barceló A, de la Peña M, Gozal D, Agustí A, Barbé F. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J 2007;30(1):110–13. [DOI] [PubMed] [Google Scholar]
- 8.Sharkey KM, Orff HJ, Tosi C, Harrington D, Roye GD, Millman RP. Subjective sleepiness and daytime functioning in bariatric patients with obstructive sleep apnea. Sleep Breath 2013;17(1):267–74. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen JH, Shi L, Mokhlesi B. Factors associated with excessive daytime sleepiness in patients with severe obstructive sleep apnea. Sleep Breath 2013;17(2):629–35. [DOI] [PubMed] [Google Scholar]
- 10.Pamidi S, Knutson KL, Ghods F, Mokhlesi B. Depressive symptoms and obesity as predictors of sleepiness and quality of life in patients with REM-related obstructive sleep apnea: cross-sectional analysis of a large clinical population. Sleep Med 2011;12(9):827–31. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Xiong KP, Lian YX, Huang JY, Zhao MY, Li JX, Liu CF. Daytime sleepiness and its determining factors in Chinese obstructive sleep apnea patients. Sleep Breath 2011;15(1):129–35. [DOI] [PubMed] [Google Scholar]
- 12.Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep 2011;34(7):859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budhiraja R, Parthasarathy S, Budhiraja P, Habib MP, Wendel C, Quan SF. Insomnia in patients with COPD. Sleep 2012;35(3):369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budhiraja R, Quan SF, Punjabi NM, Drake CL, Dickman R, Fass R. Power spectral analysis of the sleep electroencephalogram in heartburn patients with or without gastroesophageal reflux disease: a feasibility study. J Clin Gastroenterol 2010;44(2):91–6. [DOI] [PubMed] [Google Scholar]
- 15.Kushida CA, Nichols DA, Quan SF, Goodwin JL, White DP, Gottlieb DJ, Walsh JK, Schweitzer PK, Guilleminault C, Simon RDand others. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med 2006;2(3):288–300. [PubMed] [Google Scholar]
- 16.HAMILTON M A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14(6):540–5. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res 2000;9(1):5–11. [DOI] [PubMed] [Google Scholar]
- 19.Doghramji K, Mitler MM, Sangal RB, Shapiro C, Taylor S, Walsleben J, Belisle C, Erman MK, Hayduk R, Hosn R and others. A normative study of the maintenance of wakefulness test (MWT). Electroencephalogr Clin Neurophysiol 1997;103(5):554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan SF, Chan CS, Dement WC, Gevins A, Goodwin JL, Gottlieb DJ, Green S, Guilleminault C, Hirshkowitz M, Hyde PRand others. The association between obstructive sleep apnea and neurocognitive performance--the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2011;34(3):303–314B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budhiraja R, Kushida CA, Nichols DA, Walsh JK, Simon RD, Gottlieb DJ, Quan SF. Impact of Randomization, Clinic Visits, and Medical and Psychiatric Cormorbidities on Continuous Positive Airway Pressure Adherence in Obstructive Sleep Apnea. J Clin Sleep Med 2016;12(3):333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Björnsdóttir E, Benediktsdóttir B, Pack AI, Arnardottir ES, Kuna ST, Gíslason T, Keenan BT, Maislin G, Sigurdsson JF. The Prevalence of Depression among Untreated Obstructive Sleep Apnea Patients Using a Standardized Psychiatric Interview. J Clin Sleep Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab 2005;90(8):4510–5. [DOI] [PubMed] [Google Scholar]
- 24.Grandner MA, Martin JL, Patel NP, Jackson NJ, Gehrman PR, Pien G, Perlis ML, Xie D, Sha D, Weaver T and others. Age and sleep disturbances among American men and women: data from the U.S. Behavioral Risk Factor Surveillance System. Sleep 2012;35(3):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards BA, O’Driscoll DM, Ali A, Jordan AS, Trinder J, Malhotra A. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med 2010;31(5):618–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford MR, Bartlett DJ, Coughlin SR, Phillips CL, Neill AM, Espie CA, Dungan GC 2nd, Wilding JP, Calverley PM, Grunstein RRand others. The effect of continuous positive airway pressure usage on sleepiness in obstructive sleep apnoea: real effects or expectation of benefit? Thorax 2012;67(10):920–4. [DOI] [PubMed] [Google Scholar]
- 27.Nguyên XL, Rakotonanahary D, Chaskalovic J, Philippe C, Hausser-Hauw C, Lebeau B, Fleury B. Residual subjective daytime sleepiness under CPAP treatment in initially somnolent apnea patients: a pilot study using data mining methods. Sleep Med 2008;9(5):511–6. [DOI] [PubMed] [Google Scholar]
- 28.Pépin JL, Viot-Blanc V, Escourrou P, Racineux JL, Sapene M, Lévy P, Dervaux B, Lenne X, Mallart A. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J 2009;33(5):1062–7. [DOI] [PubMed] [Google Scholar]
- 29.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004;27(2):194–201. [DOI] [PubMed] [Google Scholar]
- 30.Fong SY, Ho CK, Wing YK. Comparing MSLT and ESS in the measurement of excessive daytime sleepiness in obstructive sleep apnoea syndrome. J Psychosom Res 2005;58(1):55–60. [DOI] [PubMed] [Google Scholar]
- 31.Budhiraja R, Thomas R, Kim M, Redline S. The Role of Big Data in the Management of Sleep-Disordered Breathing. Sleep Med Clin 2016;11(2):241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budhiraja R, Parthasarathy S, Drake CL, Roth T, Sharief I, Budhiraja P, Saunders V, Hudgel DW. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep 2007;30(3):320–4. [PubMed] [Google Scholar]