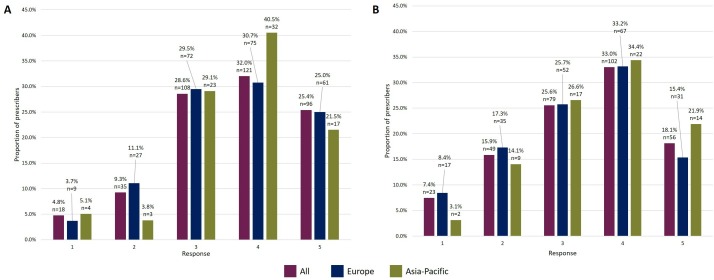
Figure 3.
Prescribers’ responses rating their level of comfort on a scale of 1–5. Prescribers’ responses, by region, when asked to rate their comfort with (A) the concept of using an EMA-approved biosimilar to treat a patient suitable for the reference biologic; (B) using an EMA-approved biosimilar in extrapolated indications that the reference biologic is approved for. EMA, European Medicines Agency.