Figure 3 |. Selection of amino acid building blocks and synthetic strategy used for the construction of the rapafucin library.
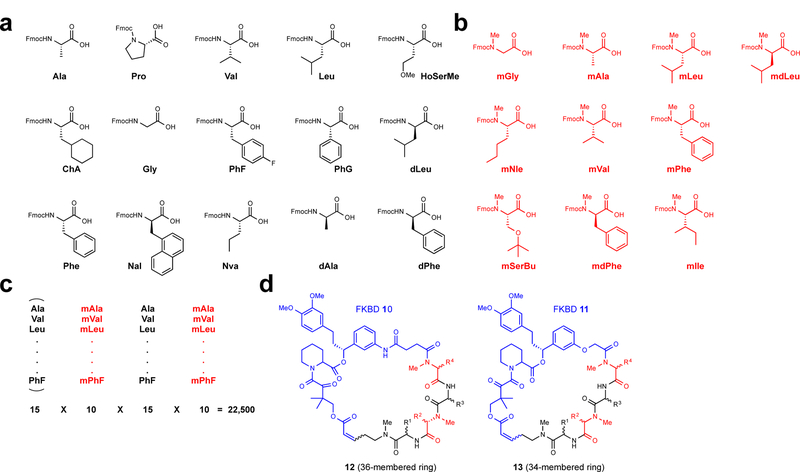
a, Fifteen N-H amino acids (black). b, Ten N-Me amino acids (red). The inclusion of N-Me amino acids was meant to increase the conformational flexibility of the effector domain and decrease susceptibility to protease degradation. c, Partial split-pool strategy for the synthesis of the rapafucin library. The first N-H amino acid building blocks are pooled whereas the rest remained split upon coupling of the individual amino acid building blocks. The pooling of the first amino acid building blocks allowed for the reduction of the total number of final products to be purified. d, Generic structure of rapafucins containing FKBD10 and FKBD11. The two FKBDs differ by two atoms, giving rise to rapafucins with 36- and 34-membered rings, respectively.
