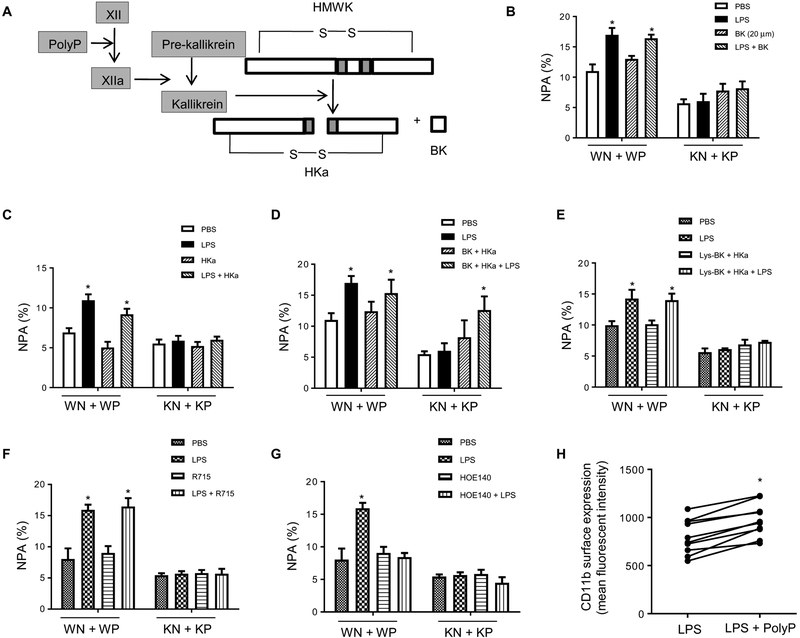
Fig. 5. PolyP regulates NPA formation through the bradykinin pathway.
(A) Schematic of polyP activation in the bradykinin pathway. HMWK (or HK) high–molecular weight kininogen; BK, bradykinin; HKa, cleaved HMWK. (B) NPA formation in the presences or absence of bradykinin (20 μM). Neutrophils were pretreated with bradykinin for 5 min and then incubated with WT or IP6K1-deficient platelets. Data are means ± SD of three experiments. *P < 0.05 versus cells treated with PBS. (C) NPA formation in the presence or absence of HKa (1 μg/ml). Data are means ± SEM of ≥3 experiments. (D) NPA formation in the presence or absence of both bradykinin and HKa. Data are means ± SEM of three experiments. (E) NPA formation in the presence or absence of HKa and Lys-BK (1 μM), a B1 receptor agonist. Data are means ± SEM of four experiments. (F) NPA formation in the presence or absence of a BK1 receptor inhibitor. Neutrophils were pretreated with BK2 receptor inhibitor R715 (8 μM) for 5 min. Data are means ± SEM of four experiments. (G) NPA formation in the presence or absence BK2 receptor inhibitor. Neutrophils were pretreated with BK2 receptor inhibitor HOE140 (150 nM) for 5 min. Data are means ± SEM of four experiments. (H) Surface expression of adhesion molecule CD11b on neutrophils. WT neutrophils and platelets were treated with polyP for 2 hours, and CD11b surface levels were detected by FACS. *P < 0.05 versus cells treated with LPS alone (n = 10 mice per group). Statistical analysis was performed using Student’s t test.