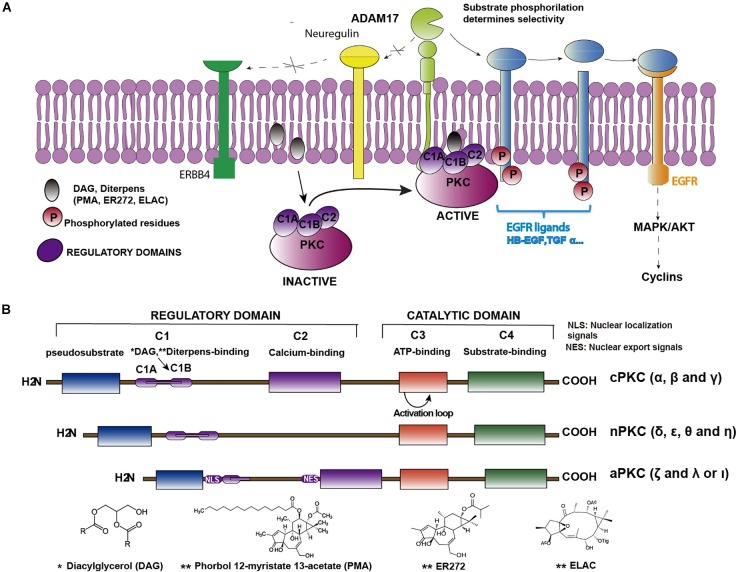
FIGURE 1.
Structure of PKC isozymes and their indirect role in EGFR activation. (A) Cartoon representing the sequence of PKC-ADAM17-TGFα-EGFR pathway. Binding of DAG or non-physiological diterpenes to the regulatory domains of PKC activates the enzymes. Upon activation, enzymes are translocated close to the plasma membrane where they catalyze the phosphorylation of membrane bound EGFR pro-ligands or ligands of other receptors of the ERBB family (i.e., neuregulin) or ligands that activate other receptors, (i.e., neuregulin, which activates ERBB4). Only phosphorylated pro-ligands are selected by ADAM17 as substrates over other non-phosphorylated ones. ADAM17-mediated shedding occurs on the phosphorylated pro-ligands, releasing the soluble ligand and activating the receptor. (B) Classification of PKC isozymes according to their structure and regulatory properties. Regulatory domains (C1 and C2) and binding sites for regulatory molecules (DAG, Ca2+, and PS) are shown as well as the conserved catalytic domains (C3 and C4). ∗See structure of Diacylglicerol below; ∗∗See structures of different diterpenes (PMA, ER272 and ELAC) below.