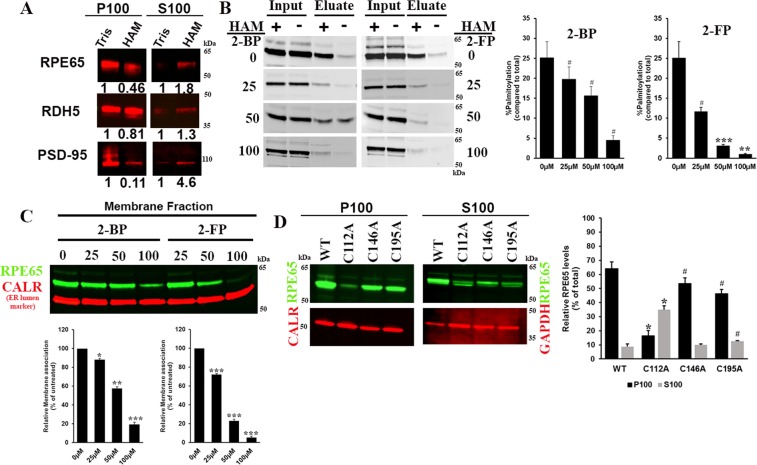
Figure 6.
Palmitoylation at C112 is crucial for RPE65-membrane interaction. (A) Bovine RPE and rod outer segments (ROS) membrane fractions were treated with 0.1 M Tris (control) or 0.5 M HAM to remove RPE65 palmitoylation, followed by membrane sedimentation. The distribution of RDH5, a non-palmitoylated transmembrane protein and PSD-95, a palmitoylated peripheral membrane protein (positive control) are indicated. P100 (membrane) and S100 (soluble) fractions were analyzed by western blotting using anti-RPE65, anti-PSD-95, and anti-RDH5 antibody. The numbers indicate the levels normalized to control sample. HEK293F-cells overexpressing RPE65 were treated with the global protein palmitoylation inhibitors 2-bromopalmitate (top) and 2-fluoropalmitate (bottom). Suppression of RPE65 palmitoylation was analyzed by acyl-RAC assay, which showed dose-dependent decrease of RPE65 palmitoylation (B) and sucrose subcellular fractionation was performed to assess the membrane localisation (C) of RPE65 after treatment with palmitoylation inhibitor (D) Wildtype RPE65 and cysteine mutants (C112, C146 and C195) were analysed for membrane association using sucrose subcellular fractionation. Protein amount used for immunoblotting was ~20 µg. GAPDH and calreticulin were loading controls for cytosolic and membrane fractions, respectively. Samples were treated with 0.5 M hydroxylamine (HAM; indicated as “+”) or 0.5 M NaCl (indicated as “−”), respectively. Data were represented as mean ±S.D. from three independent experiments. *P < 0.001, **P < 0.001, ***P < 0.0005 and #P < 0.05 unpaired and paired student’s t-test.