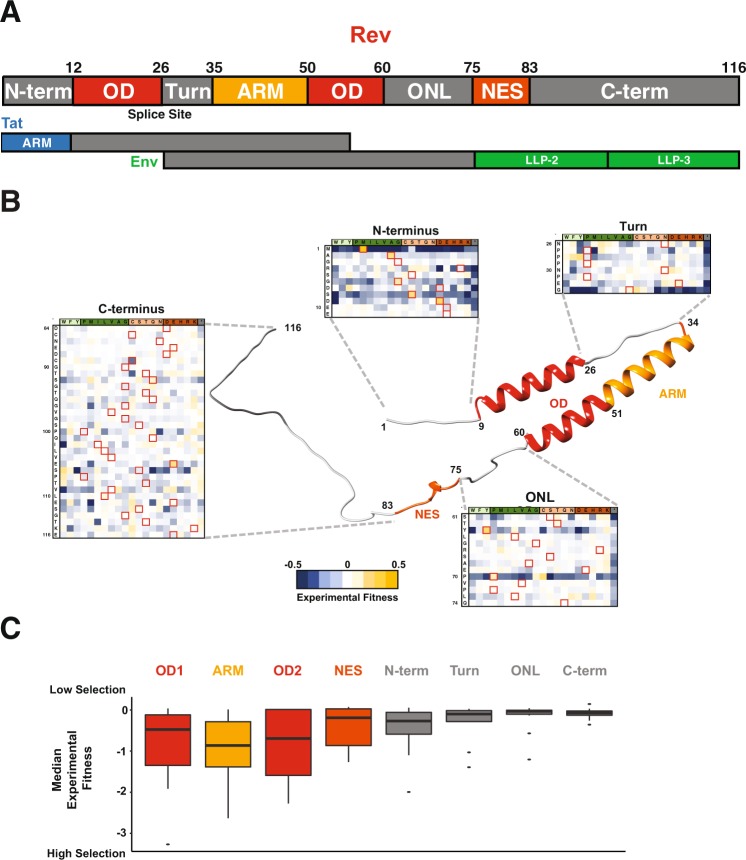
Figure 1.
Structure and Organization of HIV-1 Rev. (A) Domain organization of Rev protein (NL4-3/HXB2 numbering) with interaction surfaces in shades of red (OD: Oligomerization domain; ARM: Arginine-Rich Motif; NES: Nuclear Export Sequence) and linker regions in grey (N-term: N-terminus, Turn, ONL: OD-NES Linker). In the viral genome, the Rev coding sequence consists of two exons (Fig. 1A; “splice site” denotes exon-exon junction as it would occur in the mRNA) both of which are contained entirely within (alternative reading frame) exons of two other HIV genes, Tat and Env. Putative structured domains of Tat (ARM: Arginine Rich Motif) and Env (LLP: Lentivirus lytic peptide) are shown along with their overlap with Rev domains. (B) Structural model of Rev built using Rev crystal structures (residues 8–62 from PDBID: 3LPH; residues 76–83 from PDBID: 3NBZ; all other regions were built in PyMOL for visualization purposes). Also shown is the experimental fitness of every residue in the Rev linker regions (from10) (C). Distribution of median experimental fitness values of each residue for different regions of Rev classified by domain organization (data from10). Specifically, there are 116 total data points each representing a single residue of Rev, each binned into the appropriate domain; the representative value for each residue is the median fitness of all 21 side chains (and the stop codon) at that position).