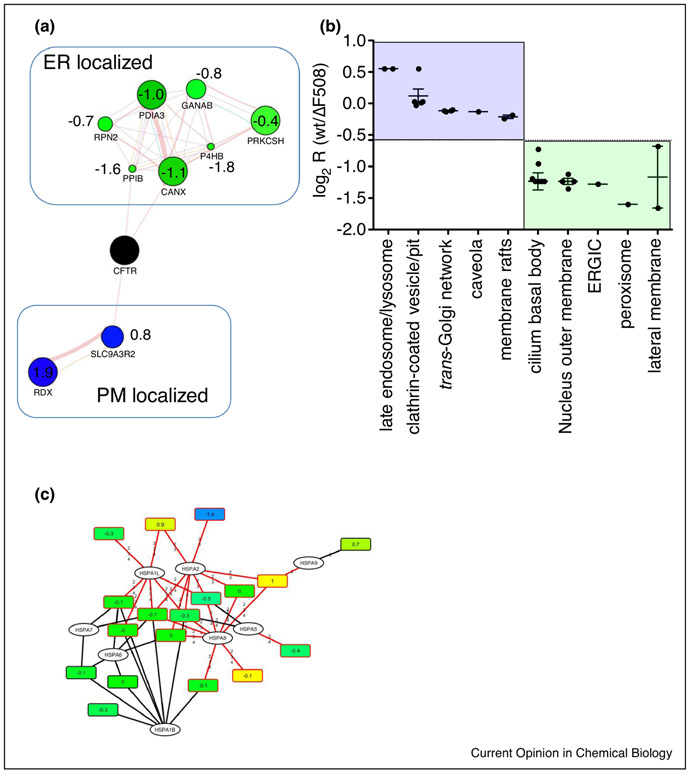
Figure 2:
A. Quantitative analysis of CFTR Co-IPs with subcellular marker proteins in a guilt-by-association approach reveals different subcellular locations for wt CFTR and the Cystic Fibrosis causing ΔF508 CFTR mutant. Blue nodes, enhanced interaction with wt CFTR; green nodes, reduced interaction with wt CFTR. Values represent log2 ratios (wt/ΔF508 CFTR). B. GO analysis using Uniprot subcellular location also reveals different subcellular locations for wt and ΔF508 CFTR, with interactors in subcellular locations associated with the plasma membrane enriched for wt CFTR. C. Bipartite protein-peptide network shows enhanced interaction of ΔF508 CFTR with HSPA2, a chaperone which initiates proteolytic processing of misfolded proteins.