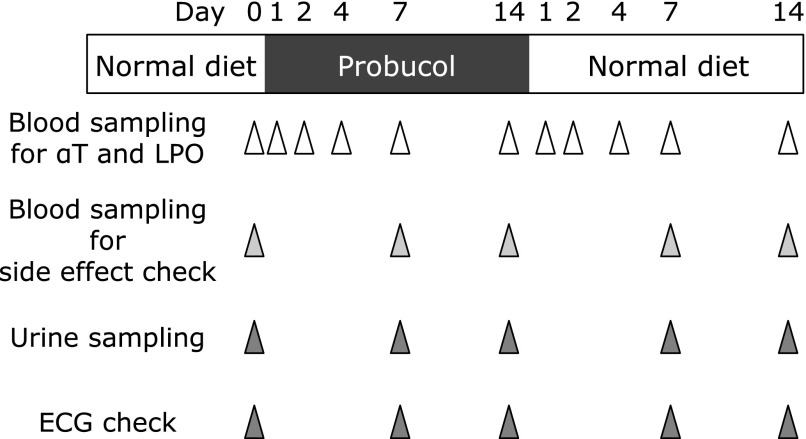
Fig. 1.
Experimental procedure for the evaluation of probucol effect. Seven- to ten-year-old male cynomolgus macaques (Macaca fascicularis), whose body weights were 6.1–8.0 kg, were divided into 2 groups randomly based on the dosage of probucol; 200 mg/kg/day probucol administration group (n = 5) and 400 mg/kg/day probucol administration group (n = 5). Probucol was orally administered by catheter twice daily for 2 weeks, after which probucol administration ceased. Plasma and erythrocyte samples for the analysis the concentrations of α-tocopherol and lipid peroxidation products were obtained at day 0, 1, 2, 4, 7, and 14 after starting probucol treatment and day 1, 2, 4, 7, and 14 after treatment cessation. Blood- and urine-sampling and ECG monitoring to evaluate adverse effects of probucol were obtained at day 0, 7, and 14 after starting probucol treatment and day 7 and 14 after cessation of treatment.