Figure 1. Single-Cell Analysis Reveals Heterogeneous, Fluctuating CDK2 Activity in S Phase.
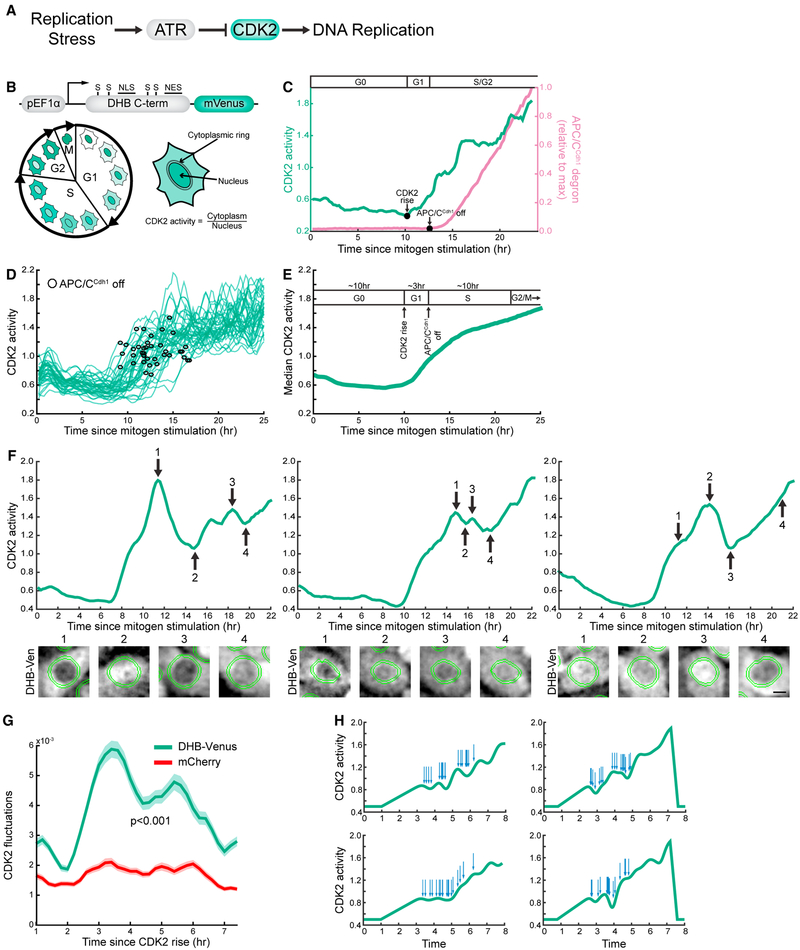
(A) Simplified model showing that activation of ATR signaling by replication stress results in CDK2 inhibition. CDK2 activity is important for many facets of S-phase progression, including the firing of new DNA replication origins.
(B) Design of the CDK2 reporter characterized in Spencer et al. (2013). C-terminal fragment of DNA helicase B (DHB) fused to mVenus. Nuclear export signal (NES), nuclear localization signal (NLS), and serine residues phosphorylated by CDK2 are indicated. Upon phosphorylation by CDK2, the reporter translocates from the nucleus to the cytoplasm. CDK2 activity can be recorded as the ratio of cytoplasmic to nuclear fluorescence intensity in live cells.
(C) Representative single-cell trace showing live-cell measurement of CDK2 activity and APC/CCdh1 degron levels. Time of CDK2 activity increase (CDK2 rise) and APC/CCdh1 inactivation are indicated. The initial rise in CDK2 activity was used to determine the transition from G0 to G1 phase. The accumulation of a fluorescently tagged anaphase-promoting complex substrate, geminin, was used to determine the time of APC/CCdh1 inactivation, which corresponds to S-phase entry.
(D) Single-cell CDK2 activity traces of 40 cells exiting quiescence upon mitogen stimulation. The time point of APC/CCdh1 inactivation is indicated on each trace with an open black circle. Computational gating was performed for cells with initially low CDK2 activity that increased over the course of imaging. One of n = 2 biological replicates.
(E) Median trace of cells that increase CDK2 activity coming out of quiescence (n > 1,000). Approximate times for each phase of the cell cycle in MCF10A cells exiting quiescence following mitogen stimulation is indicated above the averaged trace. Times are based on data presented here and in previous studies (Spencer et al., 2013; Cappell et al., 2016). One of n = 2 biological replicates.
(F) Representative single-cell CDK2 activity traces of cells exiting quiescence showing fluctuations in CDK2 activity. Raw images of the DHB-Ven construct for the indicated time points are shown beneath each trace. The cytoplasmic ring mask used for calculating the cytoplasmic signal is shown in green. Scale bar: 5 μm.
(G) Measurement of CDK2 fluctuations in single cells expressing the DHB-Ven CDK2 reporter and an untagged mCherry control following release from quiescence. Cells were computationally aligned to the time of initial CDK2 activity rise. Fluctuations were quantified by determining the difference squared between individual traces and a best-fit curve. Lines indicate mean trace and shaded regions indicate SEM (n = 590 cells). One of n = 2 biological replicates.
(H) Computational model of linearly increasing CDK2 activity traces. Twenty stress events (indicated by arrows above CDK2 trace) were simulated at random time points throughout the length of S phase. Each damage event had an equivalent inhibitory effect on CDK2 activity.
See also Figures S1 and S2.