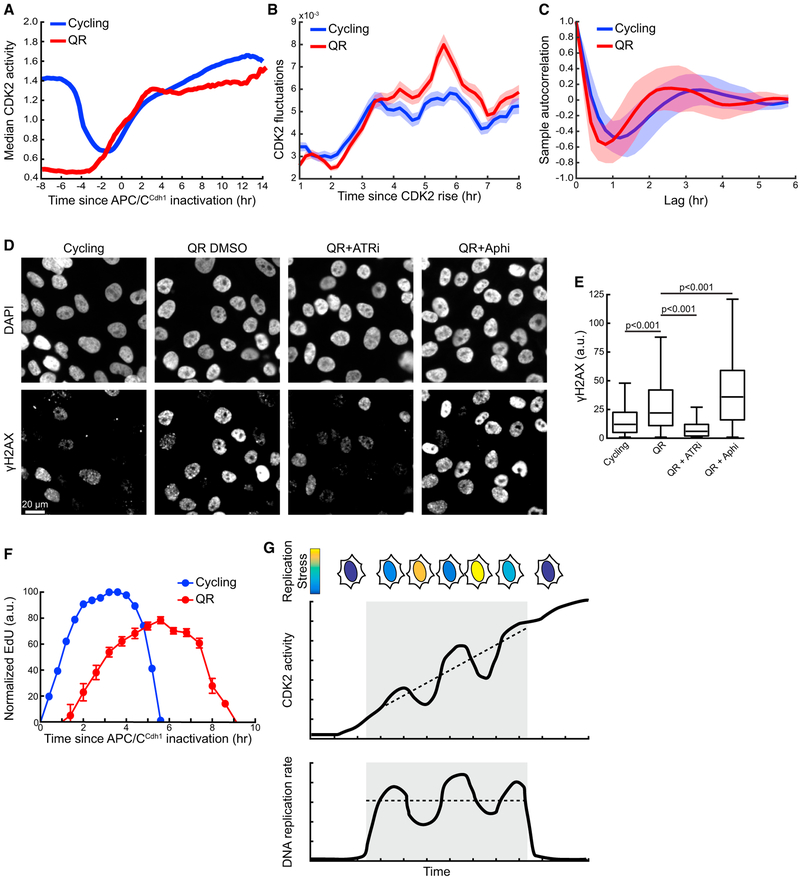
Figure 5. Increased Replication Stress in Mitogen-Induced Exit from Quiescence Causes Increased ATR-Mediated Fluctuations of CDK2 Activity and a Prolonged S Phase.
(A) Median CDK2 activity traces aligned to time of APC/CCdh1 inactivation for continuously cycling cells and quiescence-release (QR) cells stimulated with mitogens (n = 300 cells for each condition). One of n = 2 biological replicates.
(B) Fluctuation analysis of CDK2 activity in S phase of continuously cycling cells and QR (n = 1,000 cells for each condition). Lines indicate mean and shaded regions indicate SEM. One of n = 2 biological replicates.
(C) Autocorrelation analysis of S-phase CDK2 activity in cycling and QR cells (n = 300 cells for each condition). Lines indicate mean and shaded regions indicate SD. One of n = 2 biological replicates.
(D) Representative images of γH2AX staining after a 45-min incubation in DMSO, ATR inhibitor, or aphidicolin (1 μM). Scale bar, 20 μm.
(E) Boxplots showing quantification of γH2AX signal in cycling or QR cells treated with DMSO, ATR inhibitor, or aphidicolin (1 μM). Only cells in S phase at the time of drug addition were included in the analysis, as determined by APC/CCdh1 inactivation. Cycling cells were maintained in full growth media for the duration of the experiment; QR cells were stimulated with growth media 17 hr prior to drug addition. Drugs were added 45 min prior to fixation and staining (n = 449, cycling DMSO; n = 703, QR DMSO; n = 937, QR ATRi; n = 1,377, QR aphidicolin). One of n = 2 biological replicates.
(F) S-phase duration in cycling and QR cells as measured by EdU incorporation. Cells were binned by their time since APC/CCdh1 inactivation and median EdU intensity ± SEM was calculated for each bin (n > 50 cells for each bin). EdU values were normalized so that the area under the curve was the same for both conditions. One of n = 2 biological replicates.
(G) Model of how stochastic changes in the degree of replication stress cause fluctuating levels of CDK2 activity in a cell. Increases and decreases in CDK2 activity are paralleled by modulations in the rate of DNA replication.
See also Figure S5.