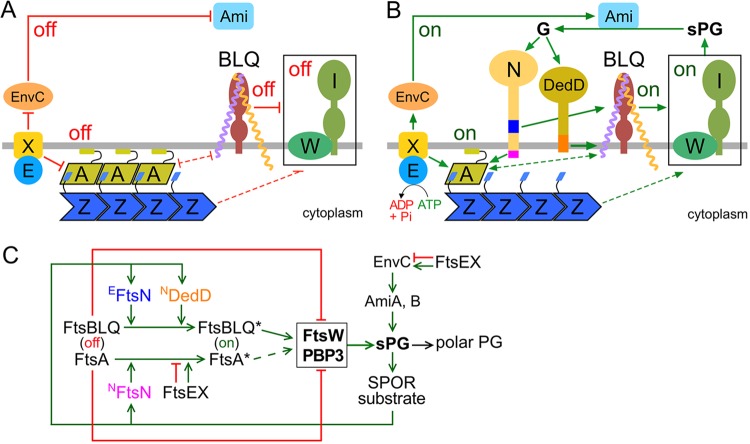
FIG 8.
Roles of DedD in stimulating E. coli cell constriction. The models are based on models proposed previously (61, 62, 71, 77, 102) and incorporate the data on DedD presented here. (A and B) Like EFtsN, NDedD stimulates FtsW-FtsI indirectly via the FtsBLQ subcomplex. For clarity, only the IM (gray line) and a relevant subset of SR proteins are depicted. FtsW (W) and PBP3 (FtsI) (I) form the core of sPG synthases (boxed) within the SR. FtsA (A) helps tether FtsZ (Z) polymers to the cytoplasmic face of the IM. FtsB (purple), FtsL (yellow), and FtsQ (brown) form the transmembrane FtsBLQ subcomplex (BLQ). FtsX (X) forms a transmembrane subcomplex with the cytoplasmic ATPase FtsE (E). EnvC and the murein amidases AmiA and AmiB (Ami) reside in the periplasm. Both FtsN (N) and DedD are bitopic IM proteins with a periplasmic C-terminal SPOR domain. Initiation of cell constriction requires inactive sPG synthases to become active. This switch is controlled by FtsA and the FtsBLQ subcomplex, each of which can adopt conformational states that either suppress (off in panel A), or allow/stimulate (on in panel B) FtsW-FtsI activity. The FtsA off (more polymeric) and FtsA on (less polymeric) conformations likely correspond to the oligomeric state of the protein. The state of FtsBLQ is assumed to be communicated to FtsW-FtsI via direct interactions between the two subcomplexes. Either state of FtsA (off or on) may help to stabilize the corresponding state of FtsBLQ, and vice versa, via direct or indirect interactions (61). The state of FtsA could be communicated to FtsW-FtsI indirectly via such effects on FtsBLQ (double-headed dashed lines). Alternatively, FtsW-FtsI senses the state of FtsA directly or via some other route (single-headed dashed lines). FtsX interacts with both FtsA and EnvC and promotes either the on or off conformations of both proteins, depending on whether FtsEX is actively hydrolyzing ATP or not, respectively. The state of EnvC, in turn, directly regulates whether the murein amidases AmiA and AmiB (Ami) are active (EnvC on) or not (EnvC off). (A) Prior to the initiation of active cell fission, both FtsA and FtsBLQ exist mostly in their off conformations. FtsEX ATPase activity is low and/or uncoupled from conformational changes in its binding partners, favoring the off states of both FtsA and EnvC. No sPG is produced, and AmiA and AmiB are inactive. (B) Both FtsN and DedD allosterically promote FtsBLQ to switch to its on conformation. While FtsN does so in the periplasm via its essential EFtsN peptide (blue), DedD does so within or very near the membrane via NDedD (orange). In the cytoplasm, meanwhile, NFtsN (magenta) directly binds FtsA and stimulates the FtsA on state. FtsEX ATPase activity leads to further stimulation of this state and also promotes the EnvC on conformation. Synthesis of the sPG annulus (sPG) is initiated, and AmiA and -B become active. As FtsW-FtsI adds new material to the inner edge of the sPG annulus, the amidases split its outer edge to generate the polar PG that will shape the two nascent cell poles. Amidase action also results in a high local concentration of denuded glycan strands (G), which are the preferred binding substrate of the SPOR domains of both FtsN and DedD. Hence, additional FtsN and DedD molecules accumulate at the SR, and a positive-feedback loop in the cell constriction process (sPG loop) is established. (C) Proposed roles of DedD within the sPG loop in schematic format. Factors that promote or inhibit progress through the loop are indicated by green or red lines, respectively. FtsEX either inhibits (ATPase inactive) or promotes (ATPase active) progress. See above and the text for further explanation.