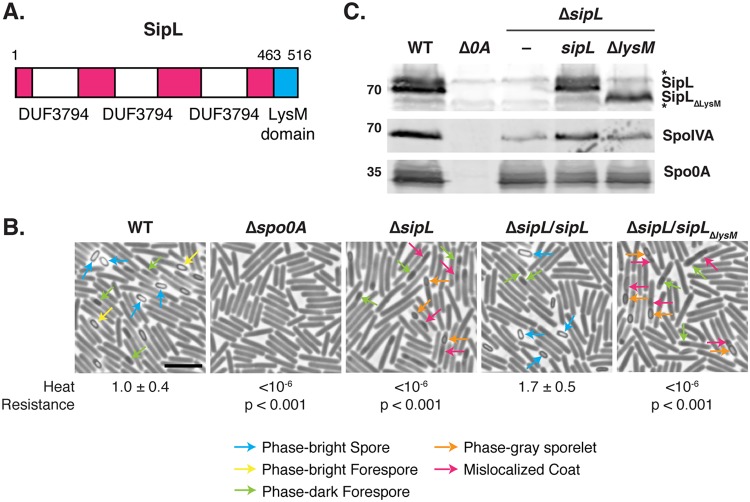
FIG 1.
The LysM domain of C. difficile is essential for spore formation. (A) Schematic of C. difficile SipL domain structure. SipL contains three domains of unknown function (DUF3794) and a C-terminal LysM domain. (B) Phase-contrast microscopy analyses of the indicated C. difficile strains ∼20 h after sporulation induction. The ΔsipL strain was complemented with either the wild-type allele or one encoding a deletion of the LysM domain (ΔsipL/sipLΔlysM strain). Arrows mark examples of sporulating cells at different stages of maturation: blue arrows highlight phase-bright free spores; yellow arrows mark mature phase-bright forespores, which are formed following cortex formation (22, 24); green arrows highlight immature phase-dark forespores; orange arrows highlight phase-gray sporelets, which produce a phase-dark ring surrounding the forespore but do not become phase-bright; and pink arrows demarcate regions suspected to be mislocalized coat based on previous studies (26, 30). Heat resistance efficiencies were determined from 20- to 24-h sporulating cultures and represent the mean and standard deviation for a given strain relative to the level of the wild type based on a minimum of three independent biological replicates. Statistical significance relative to the level of the wild type was determined using a one-way ANOVA and Tukey’s test. Scale bar, 5 μm. The limit of detection of the assay is 10−6. (C) Western blot analyses of SipL, SpoIVA, and Spo0A. SipL was detected using an antibody raised against SipL lacking its LysM domain (i.e., SipLΔLysM). Asterisks indicate nonspecific bands detected by the SipLΔLysM antibody. SpoIVA levels were analyzed because of the prior finding that SpoIVA levels are reduced in the absence of SipL (25). Modest decreases in SipL levels were observed in the ΔsipL and ΔsipL/sipLΔlysM strains. Spo0A was used as a proxy for measuring sporulation induction (4, 25). The Western blots shown are representative of the results of three independent biological replicates.