Figure 3.
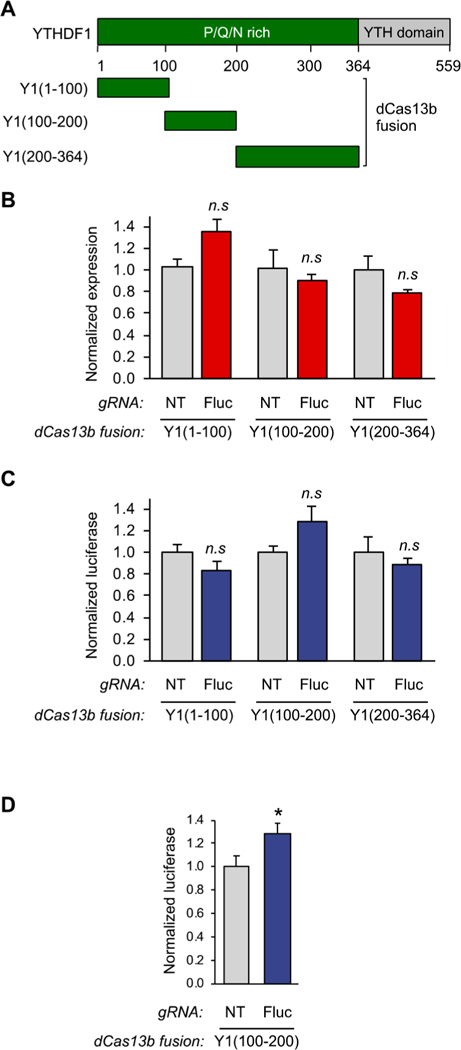
A fragment of the N-terminal domain of YTHDF1 is sufficient to bind to the translation initiation machinery. (A) Schematic diagram of YTHDF1 fragments. (B) qPCR analysis of HEK293T cells transfected with Yl(aa1–100), Yl(aa100–200), or Yl(200–364)-dCas13b fusion proteins and either the NT guide or the Fluc guide using the assay shown in Figure 2A (n = 3). (C) Protein readout analysis of the same Y1(aa1–100), Y1(aa100–200), and Y1(aa200–364) fusion proteins using the dual luciferase reporter system. The Y1(100–200) truncation shows activation of protein production without effecting RNA levels, defining this portion of the protein as the active reader domain (n = 3 biological × 2 technical). (D) Luciferase assay analysis of further replicates of the identified dCas13bY1(100–200) protein. Student’s t-test; *P < 0.03 (n = 9).
