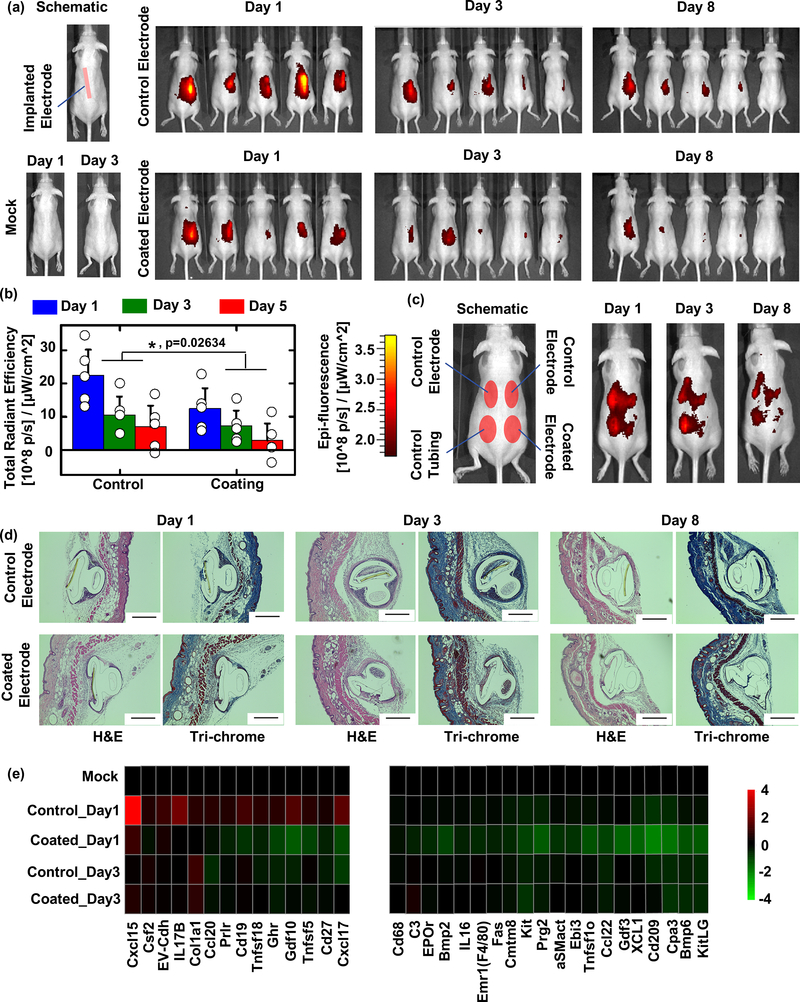
Figure 4. CGM biocompatibility in SKH1 mouse model is improved with coating.
(a) Left, schematic of subcutaneous CGM sensor implantation, and the results of mock (guide needle only and subsequent removal following) insertion into SKH1 mice for biocompatibility testing. Right, IVIS inflammation monitoring of uncoated control (top) vs. coated CGMs (bottom) after 1, 3, and 8 days post-insertion. (b) Quantification of IVIS inflammation signals from (a) and statistical analysis showed our zwitterionic coating resulted in significantly reduced inflammation at all measured time points. Data were presented as Mean±SD. * indicates statistically significant compared to the group “Control” at the level of p<0.05 using two-way ANOVA. N=5 mice/group. (c) Additional IVIS imaging was performed to examine inflammation of coated CGMs vs. both uncoated control CGMs and polyurethane tubing implanted in the same mice. (d) While fibrosis was not eliminated completely, zwitterionic coated CGMs reduced overgrowth at 1, 3, and 8 days post-insertion, as indicated by histological analysis (H&E, cellular infiltration; and Masson’s Trichrome, collagen deposition) of retrieved tissues with embedded CGMs. Scale bar: 400 μm. (e) NanoString expression analysis showing inflammation (cytokine, chemokine, and immune) markers significantly increased following 1 day of control sensor implantation and suppressed/inhibited in tissues surrounding zwitterionic-coated sensors, analyzed from tissue RNA extracts: fold changes presented on a base 2 logarithmic scale. Experiments repeated at least 2–3 times. Nanostring performed once.