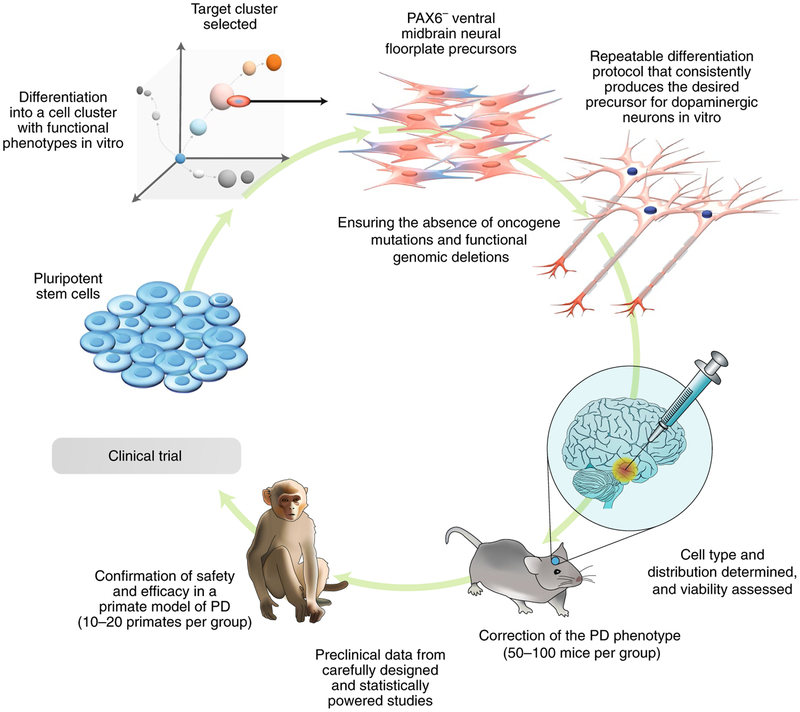
Fig. 2 |. Cell therapy for Parkinson’s disease (PD).
Pluripotent stem cells, either embryonic stem cells or induced pluripotent stem cells, can be directed towards the ventral neural floor plate lineage (PAX6−) and multiplied as dopaminergic precursors capable of the efficient production of dopamine-secreting neurons. The differentiation protocol needs to be robust and capable of being adapted for the large-scale manufacturing of a cell product that can be defined and whose biological activity is determined by dose. Product-release criteria need to be developed in order to provide consistency of function for the dose of cells to be transplanted. The selection of the cells expressing markers consistent with the ventral neural floor plate is critical for obtaining highly functioning dopamine neurons. The ability of the cells to populate the putamen and striatum and integrate into the host neural networks to produce dopamine as required is also essential. Tests for dopamine response in transplanted cells are necessary, as are for the correction of PD behavioural abnormalities (such as rotational motions). The safety and efficacy of neural transplants in the brain usually requires confirmation in a nonhuman primate PD model because rodents may insufficiently represent the human PD condition. Shown are the potential numbers of animals involved in the required preclinical studies. The target product profile and release criteria need to be developed from robust and repeatable data derived from the biological, molecular, immune and functional properties of the cells (including genomic integrity and freedom from oncogenic mutation) if the cell population is to be used for clinical trials.