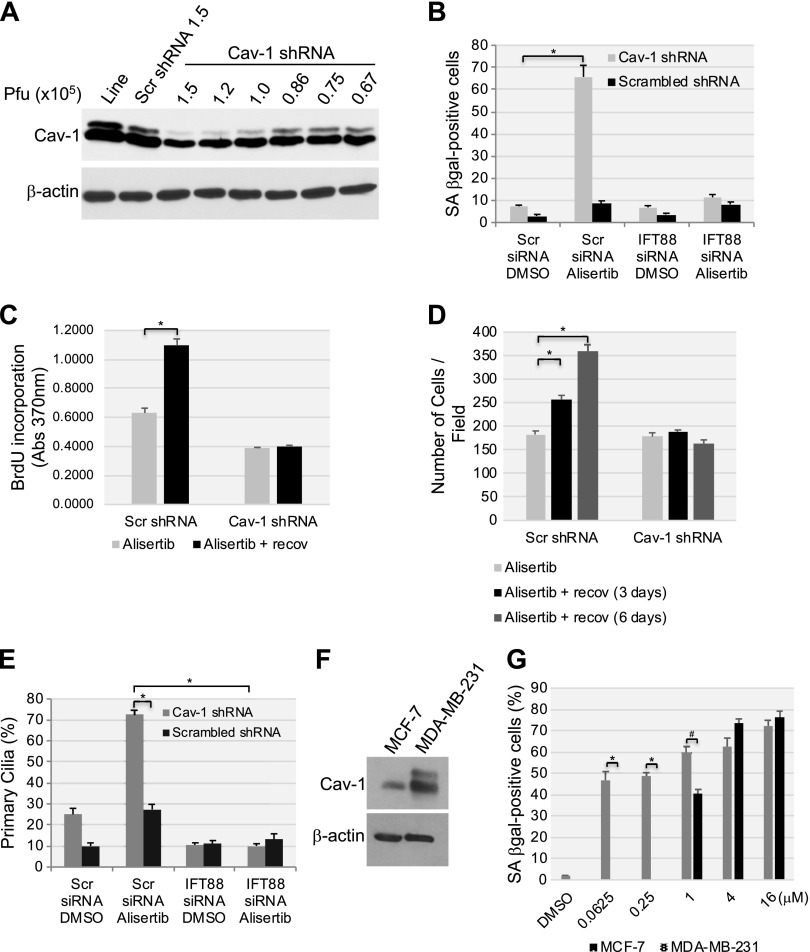
Figure 12.
Low endogenous caveolin-1 expression sensitizes human fibroblasts to alisertib-induced primary cilia formation and senescence. A) WI-38 human diploid fibroblasts were infected with a lentivirus-carrying caveolin (Cav)-1 shRNA in the indicated plaque-forming units. Infection with scrambled (Scr) shRNA and uninfected cells were used as controls. Caveolin-1 expression was determined by immunoblot analysis using anti-caveolin-1 IgGs. Immunoblot with anti-β-actin IgGs was performed as the internal control. B) WI-38 human fibroblasts were transfected with either scrambled or IFT88 siRNA. After 48 h, cells were infected with caveolin-1 shRNA at 0.86 × 105 pfu. Infection with scrambled shRNA (0.86 × 105 pfu) was performed as the control. Cells were treated with DMSO or 16 μM alisertib and cultured for 6 d. Cells were then subjected to SA-β-gal staining. C, D) WI-38 cells were transfected/infected and treated for 6 d as described in B. Cell proliferation was then quantified by BrdU incorporation assay (C) and by cell counting (D) at the end of the 6-d treatment or after the cells were washed and recovered in alisertib-free medium for an additional 6 d. E) WI-38 human diploid fibroblasts were transfected/infected and treated for 6 d, as described in B. Primary cilia formation was then quantified by immunofluorescence staining with an antibody probe specific for acetylated α-tubulin. F) Caveolin-1 expression in MCF-7 and MDA-MB-231 breast cancer cells was assessed by immunoblot analysis with a caveolin-1-specific antibody probe. Immunoblot analysis with anti-β-actin IgGs was performed to show equal loading. G) MCF-7 and MDA-MB-231 breast cancer cells were treated with different concentrations of alisertib (0.0625, 0.25, 1, 4 and 16 μM) for 6 d. Treatment with DMSO was the control. Cells were then subjected to SA-β-gal staining. Values represent means ± sem. *P < 0.001, #P < 0.005 (Student’s t test).