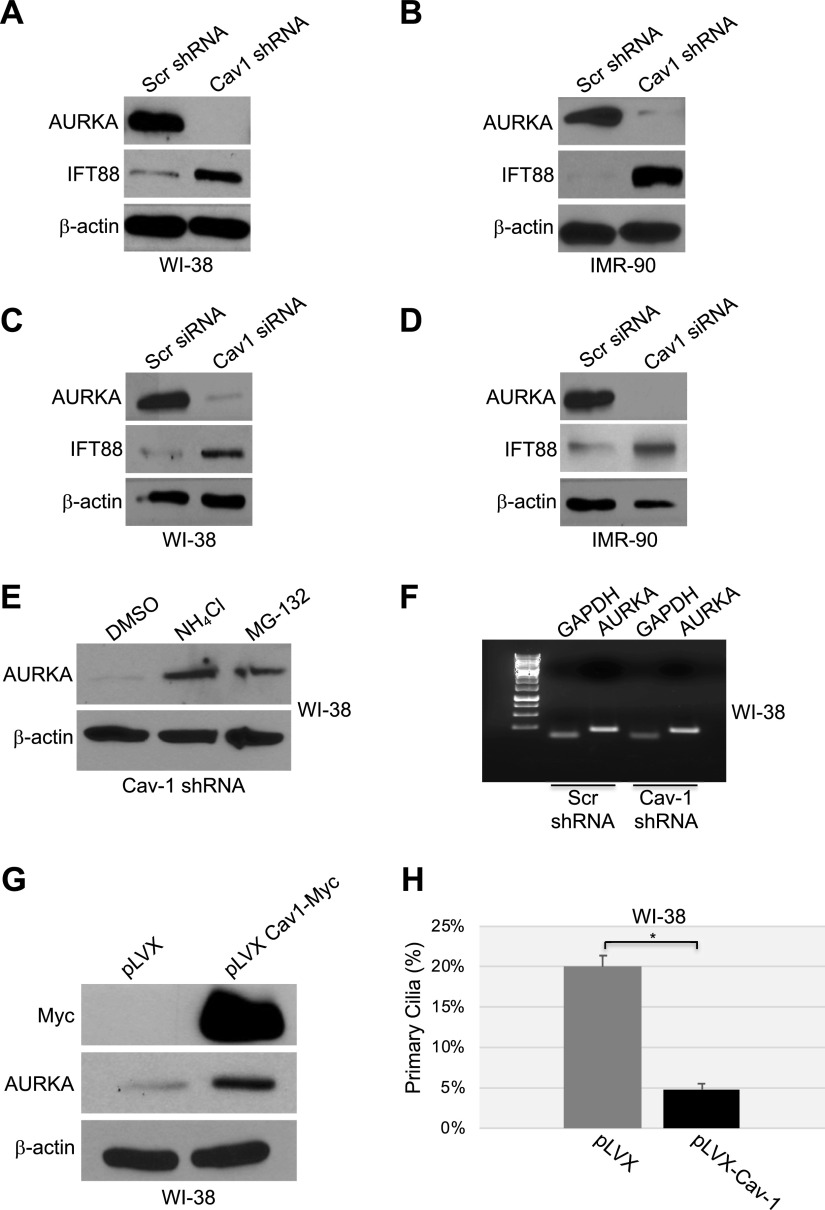
Figure 2.
A–D) Down-regulation of caveolin-1 expression promotes degradation of AURKA. WI-38 (A, C) and IMR-90 (B, D) fibroblasts were infected with a lentivirus carrying caveolin (Cav)-1 shRNA (A, B) or transfected with caveolin-1 siRNA (C, D). Infection with a lentivirus carrying scrambled (Scr) shRNA (A, B) and transfection with scrambled siRNA (C, D) were used as controls. Cells were collected after 10 d, and cell lysates were subjected to immunoblot analysis using antibody probes specific for AURKA and IFT88. Immunoblotting of anti-β-actin IgGs was performed to show equal loading. E, F) WI-38 human diploid fibroblasts were infected with caveolin-1 shRNA and cultured for 10 d in the presence or absence of ammonium chloride (NH4Cl) or the proteasome inhibitor MG-132. Infection with scrambled shRNA was performed as the control. E) Cells were collected, and the AURKA expression level was determined by immunoblot analysis with anti-AURKA IgGs. Immunoblot of anti-β-actin IgGs was performed as the control. F) Cells were collected, and AURKA mRNA level was determined by RT-PCR with specific primers. RT-PCR with primers specific for GAPDH was performed as an internal control. G, H) WI-38 fibroblasts were infected with a lentiviral vector expressing myc-tagged caveolin-1. Infection with the empty vector pLVX was performed as the control. G) After 6 d, cells were collected, and the AURKA protein level was determined by immunoblot analysis with an antibody probe specific for AURKA. Immunoblot with anti-c-myc IgGs was performed to determine exogenous caveolin-1 expression. Immunoblot of anti-β-actin IgGs was performed to show equal loading. H) Primary cilia formation was quantified by immunofluorescence analysis with anti-acetylated α-tubulin IgGs 6 d after infection. Values represent means ± sem. *P < 0.001 (Student’s t test).