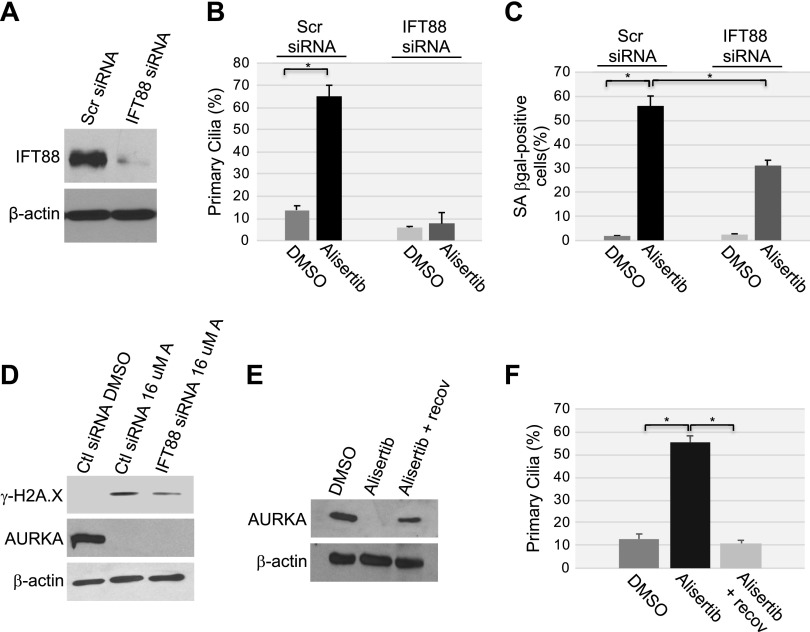
Figure 8.
The inability to reabsorb the primary cilium contributes to cellular senescence induced by alisertib. A) WI-38 human diploid fibroblasts were transfected with either scrambled (Scr) or IFT88 siRNA. Cells were collected, and cell lysates were subjected to immunoblot analysis using anti-IFT88 IgGs. Immunoblot with anti-β-actin IgGs was performed to show equal loading. B–D) WI-38 fibroblasts were transfected with IFT88 siRNA. Transfection with scrambled (Scr) siRNA was used as the control. After 48 h, cells were treated with 16 μM alisertib and cultured for 10 d. Treatment with DMSO was used as the control. B) Primary cilia formation was quantified by immunofluorescence staining with anti-acetylated α-tubulin. C) Cells were subjected to SA-β-gal activity staining. D) The expression levels of phosphorylated histone H2A.X (γ-H2A.X), AURKA, and β-actin were determined by immunoblot analysis using antibody-specific probes. E, F) WI-38 fibroblasts were treated with DMSO or alisertib (16 μM) for 10 d. Cells were then washed and recovered in alisertib-free medium for an additional 5 d. E) Cell lysates were subjected to immunoblot analysis with anti-AURKA IgGs. Immunoblot with anti-β-actin IgGs was performed to show equal loading. F) Primary cilia formation was quantified by immunofluorescence staining with anti-acetylated α-tubulin. Values in B, C and F represent means ± sem. *P < 0.001 (Student’s t test).