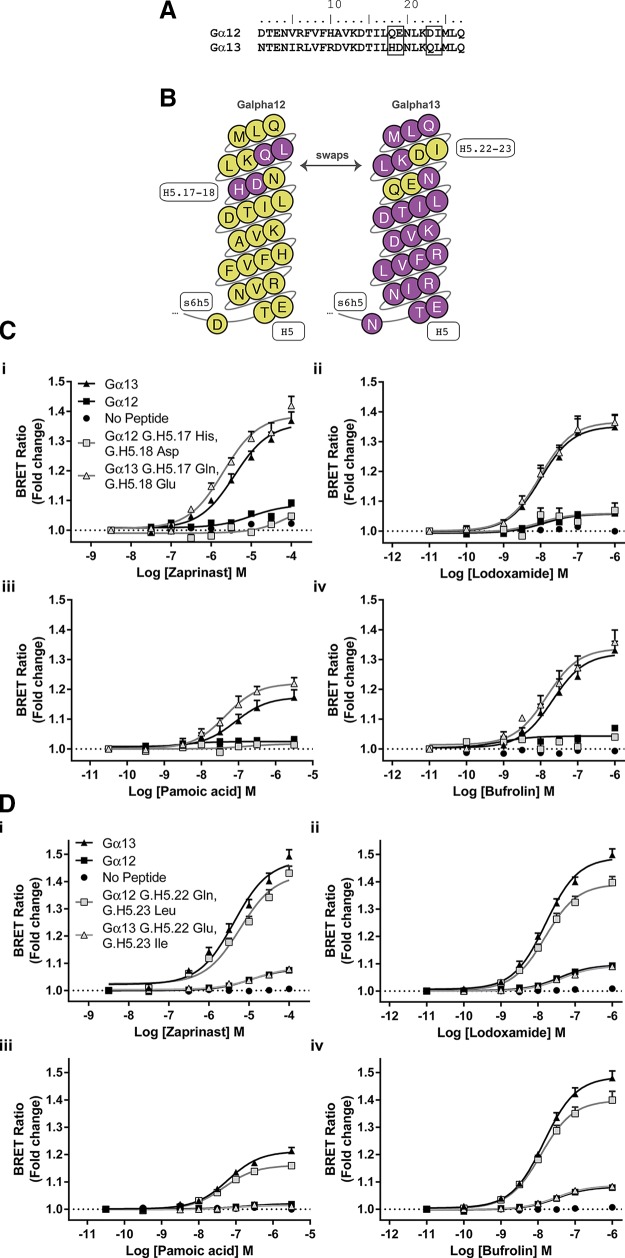
Figure 4.
G12/G13 selectivity for hGPR35 resides within the C-terminal 10 aa. A) The C-terminal 27 aa of Gα12 and Gα13 are shown with amino acids that differ within the last 10 aa boxed. B) The Common Gα Numbering system (36, 37) is used to highlight these differences and their positions within the C-terminal G protein α5 helix. C, D) hGPR35-SPASM sensors were constructed in which residues at positions G.H5.17 and G.H5.18 (C) or G.H5.22 and G.H5.23 (D) were swapped between Gα12 and Gα13. Following stable expression and induction in Flp-In T-REx 293 cells, the ability of each of zaprinast (i), lodoxamide (ii), pamoic acid (iii), and bufrolin (iv) to enhance BRET signals was compared with the effect of these ligands at the hGPR35-Gα13, hGPR35-Gα12, and hGPR35-NP sensors.