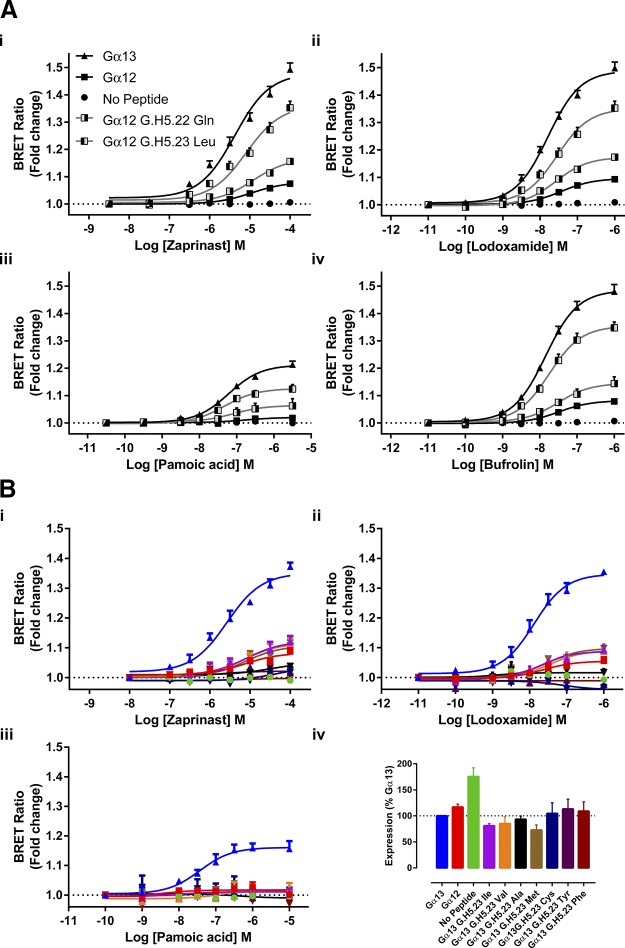
Figure 5.
G12/G13 selectivity for hGPR35 is defined predominantly by a single leucine/isoleucine variation. A) In experiments akin to Fig. 4, hGPR35-G protein sensors were constructed and expressed in which single amino acids at positions G.H5.22 and G.H5.23 were swapped between Gα12 and Gα13, and the effect of the denoted ligands [zaprinast (i), lodoxamide (ii), pamoic acid (iii), and bufrolin (iv)] were compared with results generated using the hGPR35-Gα13, hGPR35-Gα12, and hGPR35-NP sensors. B) Position G.H5.23 (Leu) in Gα13 was altered to a number of other amino acids, and functionality was assessed in response to the noted GPR35 agonists [zaprinast (i), lodoxamide (ii), and pamoic acid (iii)], with parallel responses of hGPR35-Gα13, hGPR35-Gα12, and hGPR35-NP sensors recorded as controls. Direct measures of luciferase activity in cells transiently expressing these constructs defined their relative expression levels (iv).