Key Points
Question
Is midlife diet associated with subsequent risk for dementia?
Findings
In this prospective cohort study of 8225 participants without dementia, diet quality during midlife (mean age, 50 years) was assessed using the Alternate Health Eating Index (score range, 0-110) was not significantly associated with subsequent risk for dementia (hazard ratio for each 10-point increment in Alternate Health Eating Index, 0.97).
Meaning
Repeat assessments of diet quality during midlife showed no significant association with subsequent dementia risk.
Abstract
Importance
Observational studies suggest that diet is linked to cognitive health. However, the duration of follow-up in many studies is not sufficient to take into account the long preclinical phase of dementia, and the evidence from interventional studies is not conclusive.
Objective
To examine whether midlife diet is associated with subsequent risk for dementia.
Design, Setting, and Participants
Population-based cohort study established in 1985-1988 that had dietary intake assessed in 1991-1993, 1997-1999, and 2002-2004 and follow-up for incident dementia until March 31, 2017.
Exposures
Food frequency questionnaire to derive the Alternate Healthy Eating Index (AHEI), an 11-component diet quality score (score range, 0-110), with higher scores indicating a healthier diet.
Main Outcome and Measures
Incident dementia ascertained through linkage to electronic health records.
Results
Among 8225 participants without dementia in 1991-1993 (mean age, 50.2 years [SD, 6.1 years]; 5686 [69.1%] were men), a total of 344 cases of incident dementia were recorded during a median follow-up of 24.8 years (interquartile range, 24.2-25.1 years). No significant difference in the incidence rate for dementia was observed in tertiles of AHEI exposure during 1991-1993, 1997-1999 (median follow-up, 19.1 years), and 2002-2004 (median follow-up, 13.5 years). Compared with an incidence rate for dementia of 1.76 (95% CI, 1.47-2.12) per 1000 person-years in the worst tertile of AHEI (lowest tertile of diet quality) in 1991-1993, the absolute rate difference for the intermediate tertile was 0.03 (95% CI, −0.43 to 0.49) per 1000 person-years and for the best tertile was 0.04 (95% CI, −0.42 to 0.51) per 1000 person-years. Compared with the worst AHEI tertile in 1997-1999 (incidence rate for dementia, 2.06 [95% CI, 1.62 to 2.61] per 1000 person-years), the absolute rate difference for the intermediate AHEI tertile was 0.14 (95% CI, −0.58 to 0.86) per 1000 person-years and for the best AHEI tertile was 0.14 (95% CI, −0.58 to 0.85) per 1000 person-years. Compared with the worst AHEI tertile in 2002-2004 (incidence rate for dementia, 3.12 [95% CI, 2.49 to 3.92] per 1000 person-years), the absolute rate difference for the intermediate AHEI tertile was −0.61 (95% CI, −1.56 to 0.33) per 1000 person-years and for the best AHEI tertile was −0.73 (95% CI, −1.67 to 0.22) per 1000 person-years. In the multivariable analysis, the adjusted hazard ratios (HRs) for dementia per 1-SD (10-point) AHEI increment were not significant as assessed in 1991-1993 (adjusted HR, 0.97 [95% CI, 0.87 to 1.08]), in 1997-1999 (adjusted HR, 0.97 [95% CI, 0.83 to 1.12]), or in 2002-2004 (adjusted HR, 0.87 [95% CI, 0.75 to 1.00]).
Conclusions and Relevance
In this long-term prospective cohort study, diet quality assessed during midlife was not significantly associated with subsequent risk for dementia.
This Whitehall II cohort study examines whether midlife diet assessed using the Alternate Healthy Eating Index, a food frequency questionnaire, is associated with subsequent risk for dementia in UK civil servants.
Introduction
The number of dementia cases (estimated at 47 million in 2015) is expected to triple during the next 30 years.1 In the absence of a cure, prevention is crucial and modifiable exposures during midlife are increasingly emphasized in guidelines for the prevention of dementia.2 The role of specific nutrients in brain function,3 and the fact that diet is potentially modifiable, has led to research on the association between dietary factors and cognitive aging.
Numerous studies have examined cognitive outcomes in relation to a single nutrient or food item. However, there are conceptual and methodological limitations to these studies. Nutrient interactions may be complex; the independent effect of each nutrient or food is difficult to assess; and because the causes of dementia are multifactorial, the association with a single food or nutrient may be too small to be detected reliably.4 Therefore, research increasingly focuses on an overall diet approach.5 Several studies have reported an association between overall diet and cognitive decline,6 brain imaging biomarkers,7,8 and dementia risk.9
Despite several systematic reviews and meta-analyses of observational studies during recent years,6,9 no firm conclusions can be drawn. Interventional studies on diet modification, supplementation, or both, have not shown an effect,10,11,12,13 with 1 exception.14 Most previous studies on diet and dementia had a short follow-up (typically <10 years) and reverse causation may bias the results because dietary behavior and nutrient intake may be altered during the long preclinical period of dementia.15
This study examined the association between midlife diet quality using the Alternative Healthy Eating Index (AHEI) and dietary patterns and subsequent incidence rates for dementia and cognitive decline.
Methods
Population
The protocol of the Whitehall II study was approved by the joint University College London and University College London Hospitals committee on the ethics of human research and was renewed at each contact period. Participants provided written informed consent at each contact in this ongoing prospective cohort study established in 1985-1988 among 10 308 civil servants (6895 men and 3413 women).16
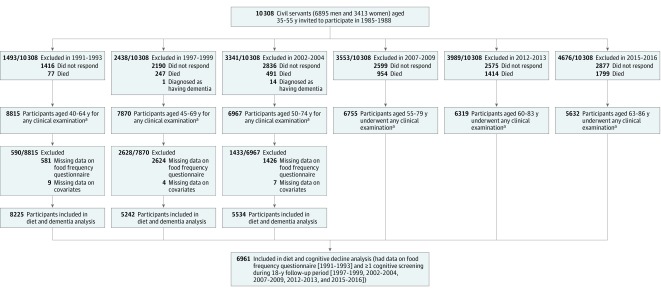
All persons aged 35 to 55 years working in 20 London-based departments were invited to participate by letter and 73% agreed. Since baseline, follow-up clinical examinations have taken place approximately every 5 years during 1991-1993, 1997-1999, 2002-2004, 2007-2009, 2012-2013, and 2015-2016 (Figure 1).
Figure 1. Flowchart of Sample Selection for Whitehall II Study.
The end of follow-up for dementia occurred on March 31, 2017. There was continuous linkage for dementia ascertainment throughout all of the years of follow-up.
aAt each wave of data collection, individuals participated in at least 1 part of the clinical examination (providing data from the food frequency questionnaire [1991-1993, 1997-1999, 2002-2004], from the cognitive examination [1997-1999, 2002-2004, 2007-2009, 2012-2013, 2015-2016], or on the covariates).
Diet Assessment
Dietary intake was assessed during 1991-1993, 1997-1999, and 2002-2004 using a machine-readable food frequency questionnaire (FFQ),17 which was based on the version used in the Nurses’ Health Study.18 The food list (127 items) in the FFQ was anglicized and adapted to include foods commonly eaten in the United Kingdom.19 Details of the analysis to estimate nutrients and total energy intake appear in the eMethods in the Supplement.
Participants with an incomplete FFQ (missing items for >10%) and participants with unreasonably high (>3500 kcal/d for women and >4200 kcal/d for men) or low intakes (<500 kcal/d for women and <800 kcal/d for men) were excluded to avoid the influence of outliers. The FFQ data were used to assess diet quality using the AHEI score (primary exposure) and to derive a posteriori dietary patterns (secondary exposure).
Primary Exposure
The AHEI version from 2010 was used.20 The AHEI diet score is based on 11 components: 6 components for which the highest intake is seen to be ideal (vegetables, fruits, whole grains, nuts and legumes, long-chain omega-3 fatty acids, and polyunsaturated fatty acids [excluding long-chain omega-3 polyunsaturated fatty acids]); 4 components for which avoidance or the lowest intake is seen to be ideal (sugar-sweetened drinks and fruit juice, red and processed meat, trans fat, and sodium); and 1 component for which moderate consumption is thought to be ideal (alcohol intake).
The total AHEI score ranges from 0 to 110, with higher scores representing a healthier diet. Scoring criteria for the AHEI and its distribution appear in eTable 1 in the Supplement. Along with AHEI score at the 3 time points (1991-1993, 1997-1999, and 2002-2004), the mean of the 3 measures also was calculated to reduce measurement error inherent in single assessments and to reflect long-term dietary intake.
Secondary Exposure
The 127 items of the FFQ were grouped in 37 predefined food groups based on the nutrient profile and culinary use of the food item (eTable 2 in the Supplement). Dietary patterns were identified using principal component analysis (the factor procedure in SAS software version 9.4, SAS Institute Inc). Orthogonal transformation (Varimax rotation function) was undertaken to allow greater interpretability and independence of factors. Two factors were retained based on eigenvalues, the Scree plot, the interpretability of factors, and the percentage of variance explained by the factors.
The first pattern was termed the healthy food diet and had high intake of vegetables, fruits, and fish. The second pattern was termed the Western-type diet and had high consumption of fried food, processed and red meat, pies, chocolate and sweets, high-fat dairy products, and refined grains (eTable 3 in the Supplement). Each participant received a score for both these patterns (range, −2.6 to 12.7 for the healthy food dietary pattern and −2.7 to 7.3 for the Western-type dietary pattern) based on his or her food intake. Higher scores indicate higher food intake of the particular dietary pattern.
Outcomes
The primary outcome was incident dementia and the secondary outcome was cognitive decline between 1997-1999 and 2015-2016.
Primary Outcome of Dementia
Dementia cases were identified using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes F00, F01, F02, F03, F05.1, G30, and G31.0. The National Health Service in the United Kingdom (England, Scotland, and Wales) provides most of the health care, including outpatient and inpatient care. Private medical insurance, which is held by around 11% of the UK population (according to 2014 data),21 is mainly used for elective surgery rather than for chronic conditions such as dementia.
Electronic health record linkage was undertaken using the unique National Health Service identification numbers given to each resident in the United Kingdom. The cases of dementia were ascertained via linkage to 3 electronic health record databases: the national Hospital Episode Statistics, the Mental Health Services Data Set, and the National Health Service Central Register (the British national mortality register). The date of incident dementia was set as the first record of dementia in any of the 3 databases.
Electronic health record linkage for every year of follow-up was available for all participants until March 31, 2017 (or date of death for those who died before this date). The sensitivity for dementia is 78.0% and the specificity is 92.0% for the Hospital Episode Statistics database.22 The Mental Health Services Data Set contains information on use of mental health services in hospitals, outpatient clinics, and in the community.
Secondary Outcome of Cognitive Function
A cognitive test battery was introduced to the study in 1997-1999 and repeated using the same tests at all subsequent assessments, allowing an analysis of cognitive function using 5 waves of data between 1997-1999 (age of participants was 45-69 years) and 2015-2016 (age of participants was 63-86 years). Memory was assessed using a 20-word free recall test (range of possible values, 0-20). Participants were presented a list of 1 or 2 syllable words at 2-second intervals and were then asked to recall in writing as many of the words as possible in any order within 2 minutes.
Executive function was assessed with the Alice Heim 4-I test, which is composed of a series of 65 verbal and mathematical reasoning items of increasing difficulty (score range, 0-65).23 It tests inductive reasoning by measuring the ability to identify patterns and infer principles and rules. Participants had 10 minutes to take the test.
Fluency was assessed using phonemic (score range, 0-35) and semantic (score range, 0-35) fluency tests.24 Participants were asked to recall in writing as many words beginning with “s” (phonemic fluency) and as many animal names (semantic fluency) as they could. One minute was allowed for each test. The tests have good test-retest reliability (score range, 0.6-0.9) and were assessed in 556 participants and retested within 3 months during 1997-1999.
A global cognitive score was created by first standardizing the raw scores for each cognitive domain (memory, executive function, and fluency) to z scores using a mean and standard deviation from the 1997-1999 wave to allow cognitive change from this time point to be estimated. These z scores were summed and restandardized to yield the global score (score range over the 5 assessments, −4.6 to 3.3). This approach minimizes measurement error in individual tests.25 None of these cognitive tests have recognized minimal clinically important differences.
Covariates
Sociodemographic factors consisted of age, sex, marital status, occupational position, and education level. Race/ethnicity also was included in the analyses to account for the influence of sociocultural factors on dietary habits and its association with dementia risk. It was self-reported through predefined categories and analyzed as a binary variable using the categories of white vs nonwhite (South Asian, black, or other) to allow sufficient numbers in each group. Total energy intake was derived from the FFQ.
Health behavioral factors consisted of smoking status, alcohol consumption, and hours of moderate to vigorous physical activity per week. Health factors included hypertension, dyslipidemia, type 2 diabetes, body mass index, coronary heart disease or stroke, taking medications for cardiovascular disease, depressive symptoms, and apolipoprotein E ε4 genotype. Details on the assessment and categorization of covariates appear in the eMethods in the Supplement.
Statistical Analysis
All analyses were exploratory. The sensitivity analyses were performed post hoc and only undertaken for the primary outcome of dementia. Analyses on the association between diet and cognitive decline (secondary outcome) aimed to assess the similarity in the associations between dementia and cognitive decline.
The dietary quality scores assessed by the AHEI (primary exposure) and the dietary pattern scores (secondary exposure) were grouped into tertiles to allow sufficient numbers of dementia cases in each category. The tertiles also were modeled as continuous variables and 3 categories were used (estimations for a 1-SD [10-point] increment in the AHEI score, a 1-SD increment in the healthy food dietary pattern, and a 1-SD decrement in the Western-type dietary pattern) so that all estimates corresponded with associations of better diet quality. Three sets of analyses were undertaken and are described below.
Association Between Diet and Incidence Rate for Dementia
Cox proportional hazard models with age as the timescale were used to examine associations between diet and risk for incident dementia. Participants were censored at the recorded date of dementia, death (to account for the competing risk of mortality), or March 31, 2017, whichever came first. These analyses were undertaken using scores on diet (AHEI and dietary patterns) and covariates from 1991-1993, 1997-1999, and 2002-2004 in 3 separate analyses.
The proportional hazards assumption test was verified using the Schoenfeld residuals test. The association between mean AHEI (from 3 assessments) and incidence of dementia also was examined with follow-up for dementia starting in 2002-2004 in these analyses. All analyses were first adjusted for age, sex, race/ethnicity, and total energy intake, then for sociodemographic and behavioral factors, and then for health covariates. Among participants with data on apolipoprotein E ε4 genotype, an additional adjustment for this covariate was undertaken.
Trajectories of AHEI Before Dementia Diagnosis
Linear mixed-effect models26 with random intercept and slope were used to examine trajectories of AHEI from 1991-1993 to 2002-2004 among participants who developed dementia subsequent to the last measure of AHEI compared with those who were free of dementia at the end of follow-up (March 2017 or date of death). Dietary scores were the dependent variable in these analyses. The models used all available data during follow-up, handled differences in length of follow-up, and took into account the fact that repeated measures on the same individual are correlated.
The model included time since 1991-1993, time squared, age during 1991-1993 (centered on a mean age of 50 years), sex, race/ethnicity, incident dementia (coded as 1 for cases and 0 for all others), time since 1991-1993, time squared, and their interactions with slope terms (time and time squared). Estimation of the differences in AHEI scores between incident dementia cases and noncases for each year during follow-up was undertaken using the MARGINS command in Stata version 15.1 (StataCorp).
Association Between Diet and Subsequent Cognitive Decline
Linear mixed-effect models with intercept and slope fitted as random effects were used to examine the association among diet assessed in 1991-1993, cognitive function in 1997-1999, and subsequent 18-year cognitive decline. The basic model included the following terms: time since 1997-1999, time squared, age in 1991-1993 (centered on a mean age of 50 years), sex, race/ethnicity, total energy intake, the dietary exposure of interest, their interactions with time, and the age × time squared interaction to account for accelerated cognitive decline at older ages.
A dietary score × time interaction term was used to test the differences in cognitive decline according to tertiles of dietary exposures. The analyses were further adjusted for sociodemographic, health behavioral, and health factors and their interactions with time.
Sensitivity Analyses
Six post hoc sensitivity analyses were undertaken to assess the robustness of the findings. There was an analysis using the model by Fine and Gray, which is an alternative method to account for competing risk of death. Another analysis classified cases of dementia by 2 categories (participants with vs without cardiovascular disease history) to assess similarities in associations with diet. There was another analysis that used inverse probability weighting to account for the influence of missing data. In another analysis, the number of dementia cases was augmented by including persons with poor cognitive performance (SD <−2 or Mini-Mental State Examination score <18) as possible dementia cases to improve statistical power. There was an analysis of the association between diet and mortality to ensure validity of the dietary exposures. In the final analysis, the Mediterranean diet score was used; however, following the Mediterranean diet is not common in this UK population. Further details appear in the eMethods in the Supplement.
Apart from the factor analysis for the derivation of dietary patterns, all analyses were undertaken using Stata version 15.1 (StataCorp). Two-sided P values were used with an α level of .05 for statistical significance. There was no adjustment for multiple comparisons; therefore, the analyses should be interpreted as exploratory.
Results
Of the 10 308 dementia-free participants at study recruitment in 1985-1988, 77 died before 1991-1993, 1416 did not participate in the 1991-1993 clinical examination, 581 had missing data on the FFQ, and 9 had missing data on covariates, leading to a study population of 8225 participants for the baseline follow-up analyses in 1991-1993 (Figure 1).
The comparison between participants included and those excluded from the analyses appears in eTable 4 in the Supplement. The mean age was 50.2 years (SD, 6.1 years; range, 41-60 years) for the participants included in the analyses for dietary exposure from 1991-1993; 5686 (69.1%) were men.
For exposures during 1997-1999, the mean age was 55.9 years (SD, 6.1 years) for the 5242 dementia-free participants included in the analyses. During 2002-2004, the mean age was 61.2 (SD, 6.0) years for the 5534 dementia-free participants included in the analyses.
During 1991-1993, the mean AHEI score was 52.7 (SD, 10.0; range, 22.0-91.0); during 1997-1999, the mean AHEI score was 53.9 (SD, 10.1; range, 21.0-92.0); and during 2002-2004, the mean AHEI score was 54.2 (SD, 10.3; range, 19.5-94.0). At the end of follow-up on March 31, 2017, the mean age of the participants was 74.2 years (SD, 5.8 years).
A median follow-up of 24.8 years (interquartile range [IQR], 24.2-25.1 years; range, 0.1-25.6 years) from the 1991-1993 dietary assessment yielded a total of 344 cases of incident dementia. The mean age at dementia diagnosis was 75.6 years (SD, 5.6 years).
Cases of dementia accrued between 1995 and 2017 and 75.9% of the dementia cases were recorded between 2010 and 2017. Characteristics of the study population at the baseline follow-up during 1991-1993 according to tertiles of AHEI score and dementia status appear in Table 1.
Table 1. Baseline Follow-up Characteristics of the Study Population in 1991-1993.
| Alternative Healthy Eating Index Tertiles in 1991-1993a | Dementia Status on March 31, 2017b | ||||
|---|---|---|---|---|---|
| Worst (Range, 22.0-48.0) |
Intermediate (Range, 48.5-57.0) |
Best (Range, 57.5-91.0) |
No Dementia | Dementia | |
| No. of participants | 2778 | 2816 | 2631 | 7881 | 344 |
| Sociodemographic Factors | |||||
| Age, mean (SD), y | 49.9 (6.1) | 50.2 (6.0) | 50.4 (6.1) | 49.9 (6.0) | 55.7 (4.7) |
| Sex, No. (%) | |||||
| Men | 1982 (71.3) | 1965 (69.8) | 1739 (66.1) | 5480 (69.5) | 206 (59.1) |
| Women | 796 (28.7) | 851 (30.2) | 892 (33.9) | 2401 (30.5) | 138 (40.1) |
| Race/ethnicity, No. (%)c | |||||
| White | 2614 (94.1) | 2573 (91.4) | 2268 (86.2) | 7152 (90.8) | 303 (88.1) |
| Nonwhite | |||||
| South Asian | 66 (2.4) | 137 (4.9) | 216 (8.2) | 398 (5.1) | 21 (6.1) |
| Black | 66 (2.4) | 81 (2.9) | 113 (4.3) | 243 (3.1) | 17 (4.9) |
| Other | 32 (1.2) | 25 (0.9) | 34 (1.3) | 88 5 (1.1) | 3 (0.9) |
| Education level <high school diploma, No. (%) | 1423 (51.2) | 1281 (45.5) | 1085 (41.2) | 3593 (45.6) | 196 (57.0) |
| Low occupational position, No. (%) | 520 (18.7) | 431 (15.3) | 411 (15.6) | 1264 (16.0) | 98 (28.5) |
| Married or cohabiting, No. (%) | 2063 (74.3) | 2158 (76.6) | 2064 (78.5) | 6028 (76.5) | 257 (74.7) |
| Total energy intake, mean (SD), kcal/d | 2095 (613) | 2046 (596) | 2086 (561) | 2075 (593) | 2078 (558) |
| Health Behavioral Factors | |||||
| Moderate to vigorous physical activity, mean (SD), h/wk | 3.3 (4.0) | 3.5 (4.2) | 3.6 (4.0) | 3.5 (4.0) | 3.6 (4.5) |
| Current smoker, No. (%) | 545 (19.6) | 382 (13.6) | 213 (8.1) | 1089 (13.8) | 51 (14.8) |
| Moderate alcohol consumption, No. (%)d | 1421 (51.2) | 1879 (66.7) | 1915 (72.8) | 5021 (63.7) | 194 (56.4) |
| Health Factors, No. (%) | |||||
| Body mass index ≥30e | 329 (11.8) | 253 (8.9) | 212 (8.1) | 743 (9.4) | 51 (14.8) |
| Type 2 diabetes | 71 (2.6) | 66 (2.3) | 63 (2.4) | 182 (2.3) | 18 (5.2) |
| Dyslipidemia | 2480 (89.3) | 2480 (88.1) | 2241 (85.2) | 6889 (87.4) | 312 (90.7) |
| Hypertension | 646 (23.3) | 604 (21.5) | 497 (18.9) | 1648 (20.9) | 99 (28.8) |
| Coronary heart disease or stroke | 47 (1.7) | 70 (2.5) | 56 (2.1) | 159 (2.0) | 14 (4.1) |
| Taking medication for cardiovascular diseasef | 216 (7.8) | 253 (9.0) | 245 (9.3) | 650 (8.3) | 64 (18.7) |
| Depressive symptomsg | 386 (13.9) | 422 (15.0) | 367 (14.0) | 1117 (14.2) | 58 (16.9) |
Includes 11 components and each component is scored from 0 (worst) to 10 (best), leading to a total score that ranges from 0 (worst score) to 110 (best score), with higher scores representing a healthier diet. In 1991-1993, the mean score was 52.7 points and corresponds to a typical diet with mean intakes of 3 servings of vegetables, 2 servings of fruits, and 1.5 (for women) and 2 (for men) servings of whole grains per day, a mean intake of 2.5 g of sodium per day, 210 mg of omega-3 polyunsaturated fatty acids, and intakes of trans fat and polyunsaturated fatty acids (excluding omega-3 polyunsaturated fatty acids) corresponding to 0.4% and 5.1%, respectively, of total energy intake. The score also included per-week consumption of the following: a mean of 1.5 servings of legumes or nuts, 5 servings of red and processed meat, 4.5 glasses of soda or fruit juice, and 11 glasses (for women) and 16 glasses (for men) of alcohol. Scoring criteria and distribution appear in eTable 1 in the Supplement.
End of follow-up or date of death.
Categories to choose from only included white, South Asian, black, and other (the other category was not further defined).
Defined as 1 to 13.9 U per week for women and 1 to 20.9 U per week for men.
Calculated as weight in kilograms divided by height in meters squared.
Includes antiplatelets, diuretics, antihypertensives, lipid-lowering agents, anticoagulants, and β-blockers.
Defined based on the 4-item depression subscale of the General Health Questionnaire (total score ≥4 of 12 points) or self-reported use of antidepressants.
Association Between Diet and Incidence Rate for Dementia
There was no evidence of a significant effect modification by sex and age (all P > .17 for interaction); therefore, the analyses were undertaken without stratification. No significant difference in the incidence rate for dementia was observed by tertiles of AHEI-assessed dietary exposure during 1991-1993 (median follow-up, 24.8 years [IQR, 24.2-25.1 years; range, 0.1-25.6 years]), 1997-1999 (median follow-up, 19.1 years [IQR, 18.6-19.4 years; range, 0.1-20.0 years]), and 2002-2004 (median follow-up, 13.5 years [IQR, 13.1-14.0 years; range, 0.1-14.5 years]) (Table 2).
Table 2. Association Between Dietary Exposures and Incidence Rate for Dementia.
| Dietary Exposures in 1991-1993 | Dietary Exposures in 1997-1999 | Dietary Exposures in 2002-2004 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence Rate for Dementia per 1000 person-years | Adjusted Hazard Ratio (95% CI)a | Incidence Rate for Dementia per 1000 person-years | Adjusted Hazard Ratio (95% CI)a | Incidence Rate for Dementia per 1000 person-years | Adjusted Hazard Ratio (95% CI)a | ||||
| Age, Sex, Race/Ethnicity, and Total Energy Intake | Plus Sociodemographic, Health Behavioral, and Health Factorsb | Age, Sex, Race/Ethnicity, and Total Energy Intake | Plus Sociodemographic, Health Behavioral, and Health Factorsb | Age, Sex, Race/Ethnicity, and Total Energy Intake | Plus Sociodemographic, Health Behavioral, and Health Factorsb | ||||
| Age, mean (SD), y | 50.2 (6.1) | 55.9 (6.1) | 61.2 (6.0) | ||||||
| No. of cases/total No. | 344/8225 | 204/5242 | 192/5534 | ||||||
| Follow-up, median (IQR), y | 24.8 (24.2-25.1) | 19.1 (18.6-19.4) | 13.5 (13.1-14.0) | ||||||
| Alternative Healthy Eating Index (AHEI) Tertilesc | |||||||||
| Worst | 1.76 (1.47-2.12) | 1 [Reference] | 1 [Reference] | 2.06 (1.62-2.61) | 1 [Reference] | 1 [Reference] | 3.12 (2.49-3.92) | 1 [Reference] | 1 [Reference] |
| Intermediate | 1.80 (1.50-2.15) | 0.93 (0.72-1.20) | 0.95 (0.73-1.23) | 2.20 (1.73-2.79) | 0.92 (0.66-1.29) | 0.98 (0.69-1.38) | 2.51 (1.95-3.23) | 0.77 (0.55-1.08) | 0.81 (0.58-1.15) |
| Best | 1.81 (1.50-2.17) | 0.90 (0.69-1.17) | 0.93 (0.71-1.22) | 2.19 (1.73-2.77) | 0.91 (0.65-1.28) | 0.95 (0.67-1.35) | 2.40 (1.84-3.11) | 0.69 (0.48-0.97) | 0.73 (0.51-1.05) |
| Per 1-SD (10-point) increment (better diet) | 0.95 (0.85-1.06) | 0.97 (0.87-1.08) | 0.95 (0.82-1.09) | 0.97 (0.83-1.12) | 0.85 (0.73-0.98) | 0.87 (0.75-1.00) | |||
| Healthy Food Dietary Pattern Tertilesd | |||||||||
| Worst | 1.59 (1.31-1.93) | 1 [Reference] | 1 [Reference] | 2.18 (1.73-2.77) | 1 [Reference] | 1 [Reference] | 2.90 (2.29-3.67) | 1 [Reference] | 1 [Reference] |
| Intermediate | 1.84 (1.54-2.21) | 0.98 (0.75-1.28) | 1.01 (0.77-1.34) | 2.21 (1.75-2.79) | 0.90 (0.64-1.26) | 0.95 (0.67-1.35) | 2.71 (2.13-3.46) | 0.79 (0.56-1.12) | 0.88 (0.61-1.25) |
| Best | 1.93 (1.62-2.31) | 0.93 (0.70-1.23) | 0.97 (0.73-1.30) | 2.05 (1.61-2.61) | 0.78 (0.54-1.13) | 0.83 (0.56-1.22) | 2.42 (1.87-3.13) | 0.63 (0.43-0.93) | 0.70 (0.47-1.05) |
| Per 1-SD increment (better diet) | 0.93 (0.82-1.04) | 0.93 (0.83-1.05) | 0.84 (0.71-0.99) | 0.86 (0.72-1.02) | 0.86 (0.73-1.02) | 0.90 (0.76-1.07) | |||
| Western-Type Dietary Pattern Tertilese | |||||||||
| Worst | 1.77 (1.47-2.13) | 1 [Reference] | 1 [Reference] | 1.88 (1.46-2.42) | 1 [Reference] | 1 [Reference] | 3.00 (2.38-3.78) | 1 [Reference] | 1 [Reference] |
| Intermediate | 1.63 (1.35-1.98) | 0.84 (0.63-1.13) | 0.86 (0.64-1.16) | 2.04 (1.60-2.60) | 0.80 (0.54-1.19) | 0.80 (0.53-1.19) | 2.46 (1.90-3.17) | 0.79 (0.54-1.16) | 0.81 (0.55-1.19) |
| Best | 1.96 (1.65-2.34) | 0.96 (0.68-1.36) | 1.00 (0.70-1.43) | 2.52 (2.03-3.14) | 0.93 (0.59-1.47) | 0.96 (0.60-1.54) | 2.59 (2.02-3.32) | 0.78 (0.49-1.24) | 0.80 (0.50-1.28) |
| Per 1-SD decrement (better diet) | 0.95 (0.81-1.12) | 0.99 (0.83-1.17) | 1.00 (0.80-1.24) | 1.03 (0.82-1.30) | 0.88 (0.71-1.09) | 0.89 (0.71-1.12) | |||
Abbreviation: IQR, interquartile range.
Estimated using Cox regression models. Participants were followed up to March 31, 2017, or date of death.
Additionally adjusted for education level, occupational position, marital status, smoking status, physical activity level, alcohol consumption (apart from when AHEI score is the exposure because alcohol is one of the components of the AHEI score), hypertension, type 2 diabetes, body mass index, dyslipidemia, depressive symptoms, coronary heart disease or stroke, and use of any cardiovascular disease medication.
Worst (lowest) tertile reflects poor adherence and best (highest) tertile reflects a healthier diet. During 1991-1993, the worst tertile corresponded to a range of 22.0 to 48.0 points; intermediate tertile, 48.5 to 57.0 points; and best tertile, 57.5 to 91.0 points. During 1997-1999, the worst tertile corresponded to a range of 21.0 to 49.5 points; intermediate tertile, 50.0 to 58.0 points; and best tertile, 58.5 to 92.0 points. During 2002-2004, the worst tertile corresponded to a range of 19.5 to 49.5 points; intermediate tertile, 50.0 to 58.5 points; and best tertile, 59.0 to 94.0 points.
Best (highest) tertile reflects greater intake of vegetables, fruits, and fish (range, −2.6 to 12.7 points over the 3 assessments). During 1991-1993, the worst (lowest) tertile corresponded to a range of −2.3332 to −0.4998 points; intermediate tertile, −0.4997 to 0.2843 points; and best tertile, 0.2851 to 11.0171 points. During 1997-1999, the worst tertile corresponded to a range of −2.5729 to −0.4864 points; intermediate tertile, −0.4862 to 0.2853 points; and best tertile, 0.2858 to 7.0588 points. During 2002-2004, the worst tertile corresponded to a range of −2.2854 to −0.4674 points; intermediate tertile, −0.4669 to 0.2709 points; and best tertile, 0.2710 to 12.7459 points.
Worst (highest) tertile indicates greater intake of fried food, processed and red meat, pies, chocolate and sweets, high-fat dairy products, and refined grains (range, −2.7 to 7.3 points over the 3 assessments). During 1991-1993, the best (lowest) tertile corresponded to a range of −2.4515 to −0.5044 points; intermediate tertile, −0.5043 to 0.3401 points; and worst tertile, 0.3407 to 7.3307 points. During 1997-1999, the best (lowest) tertile corresponded to a range of −2.6795 to −0.5079 points; intermediate tertile, −0.5064 to 0.3353 points; and worst tertile, 0.3354 to 4.7024 points. During 2002-2004, the best (lowest) tertile corresponded to a range of −2.6848 to −0.5123 points; intermediate tertile, −0.5118 to 0.3423 points; and worst tertile, 0.3427 to 4.6167 points.
During 1991-1993, the incidence rate for dementia among participants in the worst tertile of AHEI (range, 22.0 to 48.0 points) was 1.76 (95% CI, 1.47 to 2.12) per 1000 person-years; among participants in the intermediate tertile of AHEI (range, 48.5 to 57.0 points), the rate was 1.80 (95% CI, 1.50 to 2.15) per 1000 person-years; and among participants in the best tertile of AHEI (range, 57.5 to 91.0 points), the rate was 1.81 (95% CI, 1.50 to 2.17) per 1000 person-years. Compared with participants in the worst AHEI tertile, the absolute rate difference for participants in the intermediate AHEI tertile was 0.03 (95% CI, −0.43 to 0.49) per 1000 person-years and for participants in the best AHEI tertile was 0.04 (95% CI, −0.42 to 0.51) per 1000 person-years.
During 1997-1999, the incidence rate for dementia among participants in the worst tertile of AHEI (range, 21.0 to 49.5 points) was 2.06 (95% CI, 1.62 to 2.61) per 1000 person-years; among participants in the intermediate tertile of AHEI (range, 50.0 to 58.0 points), the rate was 2.20 (95% CI, 1.73 to 2.79) per 1000 person-years; and among participants in the best tertile of AHEI (range, 58.5 to 92.0 points), the rate was 2.19 (95% CI, 1.73 to 2.77). Compared with participants in the worst AHEI tertile, the absolute rate difference for participants in the intermediate AHEI tertile was 0.14 (95% CI, −0.58 to 0.86) per 1000 person-years and for participants in the best AHEI tertile was 0.14 (95% CI, −0.58 to 0.85) per 1000 person-years. In the multivariable analyses, AHEI assessed during 1991-1993 and 1997-1999 was not significantly associated with subsequent dementia risk regardless of the level of adjustment for covariates (Table 2).
During 2002-2004, the incidence rate for dementia among those in the worst tertile of AHEI (range, 19.5-49.5 points) was 3.12 (95% CI, 2.49-3.92) per 1000 person-years; among participants in the intermediate tertile of AHEI (range, 50.0-58.5 points), the rate was 2.51 (95% CI, 1.95-3.23) per 1000 person-years; and among participants in the best AHEI tertile (range, 59.0-94.0 points), the rate was 2.40 (95% CI, 1.84-3.11). Compared with participants in the worst AHEI tertile, the absolute rate difference for participants in the intermediate AHEI tertile was −0.61 (95% CI, −1.56 to 0.33) per 1000 person-years and was −0.73 (95% CI, −1.67 to 0.22) per 1000 person-years for participants in the best AHEI tertile.
In the analyses of AHEI-assessed dietary exposure during 2002-2004 that were adjusted for age, sex, race/ethnicity, and total energy intake, a higher AHEI score was associated with a lower risk for incident dementia during a mean 13-year follow-up (adjusted hazard ratio [HR] per 1-SD [10-point] increment, 0.85 [95% CI, 0.73-0.98]; P = .02). This association persisted after adjustment for sociodemographic and behavioral factors (adjusted HR per 1-SD [10-point] increment, 0.86 [95% CI, 0.75-0.99], P = .04; eTable 5 in the Supplement) and became statistically nonsignificant after adjustment for measures of health (adjusted HR per 1-SD [10-point] increment, 0.87 [95% CI, 0.75-1.00]; P = .06).
Additional adjustment for apolipoprotein E ε4 genotype showed similar findings (eTable 6 in the Supplement). Analyses with mean AHEI-assessed dietary exposure (1991-1993, 1997-1999, and 2002-2004) showed no significant association with incidence rate for dementia (eTable 7 in the Supplement). In the analyses adjusted for sociodemographic factors, health behavioral factors, and health factors, no significant association was found between dietary patterns and subsequent risk for dementia (Table 2).
Trajectories of AHEI Score Prior to Dementia Diagnosis
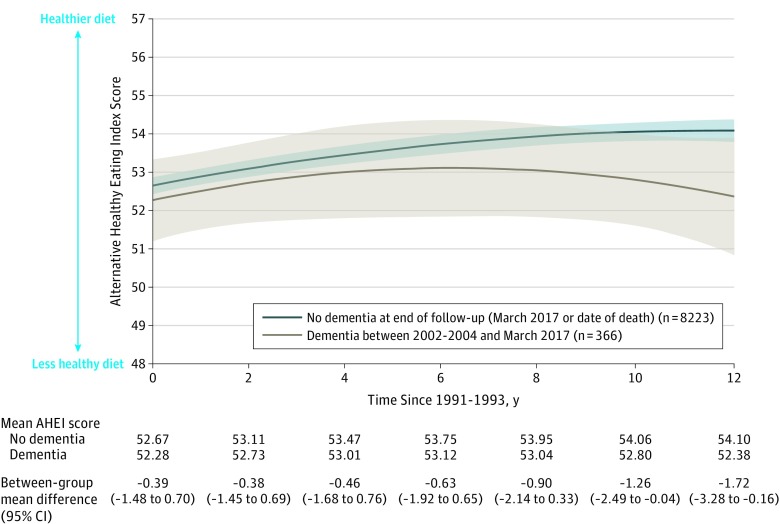
The estimation of a difference in AHEI score for each of the 12 years between 1991-1993 and 2002-2004 among participants who developed dementia between 2002-2004 and March 2017 compared with dementia-free participants showed no significant difference during the first 9 years of follow-up. However, between-group differences (dementia-free participants at end of follow-up vs participants diagnosed with dementia during the study) in AHEI score emerged at year 10 (between-group mean difference, −1.26 [95% CI, −2.49 to −0.04], P = .04; Figure 2).
Figure 2. Trajectories of Alternative Healthy Eating Index (AHEI) Score Between 1991-1993 and 2002-2004 by Incident Dementia Status.
The shaded areas represent the 95% confidence intervals (estimated using the MARGINS command in Stata 15.1). The linear mixed-effect model included time since 1991-1993, time squared, age, sex, race/ethnicity, incident dementia status, and their interactions with time and time squared. Among the patients diagnosed as having dementia, the median follow-up was between 1991-1993 and dementia was diagnosed at 20.5 years (interquartile range, 17.7-22.7 years). Among the patients without dementia, the median follow-up was 24.8 years (interquartile range, 24.4-25.2 years).
Association Between Diet and Subsequent Cognitive Decline
The analyses were undertaken on 6961 participants with cognitive data (Figure 1). The estimated mean global cognitive z scores in 1997-1999 and 18 years later by tertiles of AHEI and dietary patterns during 1991-1993 appear in eTable 8 in the Supplement. The AHEI tertiles and dietary patterns during 1991-1993 were not associated with global cognitive z scores during 1997-1999 in the fully adjusted models (Table 3).
Table 3. Association Between Dietary Exposures in 1991-1993 and Cognitive Decline From 1997-1999 to 2015-2016 (n = 6961).
| Dietary Score Range in 1991-1993 |
Adjusted Beta Coefficient (95% CI)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference in Global Cognitive z Scores During 1997-1999b | Difference in 18-y Cognitive Decline Between 1997-1999 and 2015-2016c | ||||||||
| Age, Sex, Race/Ethnicity, and Total Energy Intake | Plus Sociodemographic Factorsd | Plus Health Behavioral Factorse | Plus Health Factorsf | Adjusted for Age, Sex, Race/Ethnicity, and Total Energy Intake | Plus Sociodemographic Factorsd | Plus Health Behavioral Factorse | Plus Health Factorsf | ||
| Alternative Healthy Eating Index (AHEI) Tertilesg | |||||||||
| Worst | 22.0 to 48.0 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Intermediate | 48.5 to 57.0 | 0.10 (0.05 to 0.15) |
0.02 (−0.03 to 0.06) |
0.01 (−0.03 to 0.06) |
0.01 (−0.03 to 0.06) |
0.03 (−0.01 to 0.08) |
0.03 (−0.01 to 0.08) |
0.03 (−0.01 to 0.07) |
0.03 (−0.02 to 0.07) |
| Best | 57.5 to 91.0 | 0.14 (0.09 to 0.19) |
0.01 (−0.04 to 0.05) |
0 (−0.04 to 0.05) |
0 (−0.05 to 0.05) |
0.03 (−0.01 to 0.08) |
0.04 (−0.01 to 0.08) |
0.03 (−0.01 to 0.08) |
0.03 (−0.02 to 0.07) |
| Per 1-SD (10-point) increment (better diet) | 22.0 to 91.0 | 0.06 (0.04 to 0.08) |
0 (−0.02 to 0.02) |
0 (−0.02 to 0.02) |
0 (−0.02 to 0.02) |
0.01 (−0.01 to 0.03) |
0.01 (−0.01 to 0.03) |
0.01 (−0.01 to 0.03) |
0.01 (−0.01 to 0.03) |
| Healthy Food Dietary Pattern Tertilesh | |||||||||
| Worst | −2.3332 to −0.4998 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Intermediate | −0.4997 to 0.2843 | 0.16 (0.11 to 0.21) |
0.05 (−0.01 to 0.09) |
0.04 (−0.01 to 0.08) |
0.04 (−0.01 to 0.08) |
−0.05 (−0.09 to 0) |
−0.04 (−0.09 to 0) |
−0.05 (−0.09 to 0) |
−0.04 (−0.09 to 0) |
| Best | 0.2851 to 11.0171 | 0.21 (0.15 to 0.26) |
0.01 (−0.04 to 0.06) |
−0.01 (−0.06 to 0.04) |
0 (−0.05 to 0.01) |
−0.07 (−0.12 to −0.03) |
−0.07 (−0.12 to −0.02) |
−0.07 (−0.12 to −0.02) |
−0.06 (−0.11 to −0.01) |
| Per 1-SD increment (better diet) | −2.3 to 11.0 | 0.09 (0.07 to 0.12) |
0.01 (−0.01 to 0.03) |
0 (−0.02 to 0.02) |
0 (−0.02 to 0.03) |
−0.03 (−0.05 to −0.01) |
−0.03 (−0.05 to −0.01) |
−0.03 (−0.05 to −0.01) |
−0.03 (−0.05 to −0.01) |
| Western-Type Dietary Pattern Tertilesi | |||||||||
| Worst | 0.3407 to 7.3307 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Intermediate | −0.5043 to 0.3401 | 0.16 (0.10 to 0.21) |
0.03 (−0.02 to 0.08) |
0.04 (−0.01 to 0.09) |
0.04 (−0.01 to 0.09) |
0.04 (−0.01 to 0.09) |
0.05 (−0.01 to 0.10) |
0.04 (−0.01 to 0.09) |
0.04 (−0.01 to 0.09) |
| Best | −2.4515 to −0.5044 | 0.24 (0.17 to 0.31) |
0.02 (−0.04 to 0.08) |
0.02 (−0.04 to 0.08) |
0.02 (−0.04 to 0.09) |
0.04 (−0.02 to 0.10) |
0.05 (−0.01 to 0.11) |
0.03 (−0.03 to 0.09) |
0.03 (−0.03 to 0.09) |
| Per 1-SD decrement (better diet) | −2.5 to 7.3 | 0.14 (0.11 to 0.18) |
0.01 (−0.02 to 0.04) |
0.02 (−0.01 to 0.05) |
0.02 (−0.01 to 0.05) |
0 (−0.03 to 0.03) |
0 (−0.03 to 0.03) |
0 (−0.03 to 0.03) |
−0.01 (−0.04 to 0.02) |
Estimated using linear mixed-effect models.
The estimates for cognitive performance correspond to the beta coefficient of the diet variable at the intercept. A global cognitive score was created by first standardizing the raw scores for each cognitive domain (memory, executive function, and fluency) to z scores using a mean and standard deviation from the 1997-1999 wave of data collection to allow cognitive change from this time point to be estimated. These z scores were summed and restandardized to yield the global score (score range over the 5 assessments, −4.6 to 3.3).
The estimates for cognitive decline correspond to the coefficient of the diet variable × time interaction (rescaled to reflect cognitive decline over 18 years).
Additionally adjusted for education level, occupational position, marital status, and their interactions with time.
Additionally adjusted for smoking status, physical activity level, alcohol consumption (apart from when AHEI score is the exposure because alcohol is one of the components of AHEI score), and their interactions with time.
Additionally adjusted for hypertension, type 2 diabetes, body mass index, dyslipidemia, depressive symptoms, coronary heart disease or stroke, use of any cardiovascular disease medication, and their interactions with time.
Worst (lowest) tertile reflects poor adherence and best (highest) tertile reflects a healthier diet.
Best (highest) tertile reflects greater intake of vegetables, fruits, and fish (range, −2.6 to 12.7 points over the 3 assessments).
Worst (highest) tertile indicates greater intake of fried food, processed and red meat, pies, chocolate and sweets, high-fat dairy products, and refined grains (range, −2.7 to 7.3 points over the 3 assessments).
The mean 18-year decline in the global cognitive z score was −0.74 (95% CI, −0.76 to −0.73) of the baseline standard deviation. There were no significant associations of cognitive decline with AHEI score (P = .23 for interaction with time) or with the Western-type dietary pattern (P = .62 for interaction with time).
A higher score for the healthy food dietary pattern was associated with a greater cognitive decline (difference in 18-year cognitive decline between 1997-1999 and 2015-2016 per 1-SD increment, −0.03 [95% CI, −0.05 to −0.01]; P = .007 for interaction with time). Further analyses using the dietary indices assessed during 1997-1999 confirmed the lack of significant associations with cognitive performance and cognitive decline (eTable 9 in the Supplement).
Post Hoc Sensitivity Analyses
The analyses using the model by Fine and Gray to account for competing risk of mortality showed similar findings as the main analysis (eTable 10 in the Supplement). There were no significant associations among AHEI score and the healthy food and Western-type dietary patterns (assessed in 1991-1993, 1997-1999, and 2002-2004) and the risk for incident dementia irrespective of previous cardiovascular disease history. The exception was a reduced risk for dementia among those with a higher healthy food dietary pattern score at the 2002-2004 dietary measure and with a history of cardiovascular disease (41 of 5529 participants) (eTable 11 in the Supplement).
Inverse probability weighting to account for missing data yielded similar findings as the main analysis for all dietary pattern scores from 1997-1999 and 2002-2004; however, the association was further attenuated for AHEI score assessed in 2002-2004 (adjusted HR per 1-SD [10-point] increment, 0.90 [95% CI, 0.77-1.04], P = .16; eTable 12 in the Supplement) compared with the main analysis (adjusted HR per 1-SD [10-point] increment, 0.87 [95% CI, 0.75-1.00]; P = .06).
When the definition of dementia included possible cases, there were 785 cases among 8225 participants. In the fully adjusted models, there was no significant association among AHEI score, either of the 2 dietary patterns, and subsequent risk for dementia using this definition (eTable 13 in the Supplement). Better AHEI scores were consistently associated with lower risk for mortality irrespective of the length of follow-up (eTable 14 in the Supplement). The Mediterranean diet score was not significantly associated with the incidence rate for dementia (eTable 15 in the Supplement).
Discussion
Midlife dietary quality assessed by the AHEI score and factor analysis–derived dietary patterns was not significantly associated with subsequent risk for dementia over a median 24.8-year follow-up or with 18-year cognitive decline. This finding was supported in the analyses with repeat assessments for diet to attenuate measurement error and account for long-term dietary exposure.
Most studies on the association between diet and cognitive outcomes are based on older populations (aged ≥65 years) for which overall quality was assessed primarily using a priori dietary indices rather than a posteriori dietary patterns. Irrespective of the method used to assess overall diet,27 the findings are inconsistent.
Slower cognitive decline and reduced risk for dementia have been found with adherence to the recommended food score,6 the Dietary Approaches to Stop Hypertension (DASH),6,27 the Mediterranean-DASH Intervention for Neurodegenerative Delay diet,27 and the AHEI27 in some but not all studies.28,29,30 The Mediterranean diet is by far the index that received the most attention,6,31,32,33 and it has been shown to be associated with better cognitive outcomes in some31,33 but not all studies.6,31,32,33
Dementia has a long preclinical period involving pathophysiological changes that occur over 15 to 20 years.15 This period is characterized by accelerated cognitive decline, changes in mood, and depressive symptoms34 that are likely to affect lifestyle including dietary habits.35 Prospective studies with a follow-up of less than 10 years may be not ideally suited to examine the association of diet with cognitive dysfunction. Differences in the length of follow-up may partly explain the inconsistency in findings across the studies.
Among the few studies with follow-up longer than 10 years,28,29,36,37 there was only 1 study that used dietary patterns.38 Ozawa et al38 reported that the customary Japanese dietary pattern was associated with a reduced risk for dementia in 1006 community-dwelling Japanese participants (mean age of 68 years at dietary assessment and median follow-up of 15 years). All other studies used dietary indices.
One of the studies with follow-up longer than 10 years included 3831 participants (mean age of 74 years at dietary assessment) and showed the DASH and the Mediterranean diet scores to be associated with better cognitive performance on the modified Mini-Mental State Examination.37 In this study,37 differences in cognitive performance were maintained but not modified over the 11-year follow-up; however, the authors concluded that their study could not assess the direction of the association between diet and cognitive decline. All of the other studies did not find an association with cognitive outcomes; these studies were based on scores for the Mediterranean diet,28,29,36 the DASH diet,28 the AHEI,28 the Healthy Diet Indicator (based on World Health Organization recommendations),29 and the low carbohydrate high protein diet.29
Results from the present study are in accordance with the absence of a significant association among diet, subsequent dementia risk, and cognitive decline when the follow-up period is longer than 15 years. In this study, the association was not statistically significant when the AHEI score was assessed during late midlife and the median follow-up was less than 15 years. The present study suggested a slight decrease in diet quality in the years preceding dementia diagnosis, which also was reported in another study,35 and is compatible with the hypothesis that change in diet quality is a feature (among others) of preclinical dementia. This observation of a possible signal could be explored in further studies.
The comprehensive approach of the present study in terms of assessment of exposures and outcomes using longitudinal data constitutes a major strength. The repeated dietary assessment starting during midlife using a validated FFQ over 11 years combined with the assessment of dementia and cognitive decline over 2 decades allowed examination of the long-term association between diet and cognitive outcomes.
The lack of a significant association between early midlife diet and cognitive outcomes is unlikely to be due to poor measures of diet quality because diet was associated with mortality in the present study. Whether a healthy diet plays a role in shaping cognitive outcomes in combination with other healthy behaviors or in subgroups at increased risk for dementia remains unclear.
Limitations
This study has several limitations. First, ascertainment of dementia using linkage to electronic health records likely misses milder cases22; however, this type of ascertainment has the advantage of allowing the analyses to include all persons recruited to the study rather than only those who continue participating in cognitive assessments for dementia at follow-up visits.
Second, dietary intake was measured using an FFQ, a commonly used method in population studies, which is open to measurement errors that are common in all self-reported dietary assessments to date.4
Third, the primary exposure, the AHEI, is based on a set of specific and limited food groups and might not cover all aspects of a healthy diet and may not be adapted to the dietary habits of all populations. However, the results were similar when using the Mediterranean diet index and factor analysis–derived dietary patterns.
Fourth, as with all observational studies, it was not possible to rule out residual confounding.
Conclusions
In this long-term prospective cohort study, diet quality assessed during midlife was not significantly associated with subsequent risk for dementia.
eMethods. Food Frequency Questionnaire (FFQ) Data Derivation Procedures, Assessment and Categorization of Covariates, and Description of Sensitivity Analyses
eTable 1. Construction and Distribution of AHEI Scores in the 8225 Whitehall II Participants
eTable 2. Food Groups Used for Factor Analyses to Identify Dietary Patterns
eTable 3. Factor Loadings (≥0.40) on two Dietary Patterns Identified Using Principal Component Analysis
eTable 4. Comparison of Characteristics of Participants Included and Excluded From the Analyses
eTable 5. Association Between Dietary Exposures and Incidence of Dementia, Detailed Adjustment Models
eTable 6. Association Between Dietary Exposures and Incidence of Dementia, Additional Adjustment for APOE ɛ4 Genotype
eTable 7. Association Between Mean AHEI Score (1991-1993, 1997-1999, 2002-2004) and Incidence of Dementia (Follow-up From 2002-2004 to 2017; N Cases/ Total N=353/8268)
eTable 8. Association Between Dietary Exposures in 1991-1993 and Cognitive Function in 1997-1999 and 18 Years Later in Fully Adjusted Models (N=6961)
eTable 9. Association Between Dietary Exposures in 1997-1999 and Cognitive Decline From 1997-1999 to 2015-2016 in Fully Adjusted Models
eTable 10. Association Between Dietary Exposures and Incidence of Dementia Using Fine and Gray Model for Competing Risk of Mortality
eTable 11. Association Between Dietary Exposures and Incidence of Dementia With and Without History of Cardiovascular Disease (CVD)
eTable 12. Association Between Dietary Exposures and Incidence of Dementia Using Inverse Probability Weighting to Take Missing Data Into Account
eTable 13. Association Between Dietary Exposures and Incidence of “Possible” Dementia Defined as low Cognitive Performance or Electronic Record of Dementia Diagnosis
eTable 14. Association Between Dietary Exposures and Mortality
eTable 15. Association Between the Mediterranean Diet Score and Incidence of Dementia
References
- 1.Livingston G, Sommerlad A, Orgeta V, et al. . Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 2.Winblad B, Amouyel P, Andrieu S, et al. . Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15(5):455-532. doi: 10.1016/S1474-4422(16)00062-4 [DOI] [PubMed] [Google Scholar]
- 3.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9(7):568-578. doi: 10.1038/nrn2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3-9. doi: 10.1097/00041433-200202000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Allès B, Samieri C, Féart C, Jutand MA, Laurin D, Barberger-Gateau P. Dietary patterns: a novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr Res Rev. 2012;25(2):207-222. doi: 10.1017/S0954422412000133 [DOI] [PubMed] [Google Scholar]
- 6.van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168. doi: 10.3945/an.114.007617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbaraly T, Sexton C, Zsoldos E, et al. . Association of long-term diet quality with hippocampal volume: longitudinal cohort study. Am J Med. 2018;131(11):1372-1381.e4. doi: 10.1016/j.amjmed.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luciano M, Corley J, Cox SR, et al. . Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology. 2017;88(5):449-455. doi: 10.1212/WNL.0000000000003559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao L, Tan L, Wang HF, et al. . Dietary patterns and risk of dementia: a systematic review and meta-analysis of cohort studies. Mol Neurobiol. 2016;53(9):6144-6154. doi: 10.1007/s12035-015-9516-4 [DOI] [PubMed] [Google Scholar]
- 10.Andrieu S, Guyonnet S, Coley N, et al. ; MAPT Study Group . Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377-389. doi: 10.1016/S1474-4422(17)30040-6 [DOI] [PubMed] [Google Scholar]
- 11.Knight A, Bryan J, Wilson C, Hodgson JM, Davis CR, Murphy KJ. The Mediterranean diet and cognitive function among healthy older adults in a 6-month randomised controlled trial: the MedLey study. Nutrients. 2016;8(9):E579. doi: 10.3390/nu8090579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marseglia A, Xu W, Fratiglioni L, et al. . Effect of the NU-AGE diet on cognitive functioning in older adults: a randomized controlled trial. Front Physiol. 2018;9:349. doi: 10.3389/fphys.2018.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soininen H, Solomon A, Visser PJ, et al. ; LipiDiDiet Clinical Study Group . 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16(12):965-975. doi: 10.1016/S1474-4422(17)30332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. . Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175(7):1094-1103. doi: 10.1001/jamainternmed.2015.1668 [DOI] [PubMed] [Google Scholar]
- 15.Jack CR Jr, Knopman DS, Jagust WJ, et al. . Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol. 2005;34(2):251-256. doi: 10.1093/ije/dyh372 [DOI] [PubMed] [Google Scholar]
- 17.Brunner E, Stallone D, Juneja M, Bingham S, Marmot M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br J Nutr. 2001;86(3):405-414. doi: 10.1079/BJN2001414 [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Stampfer MJ, et al. . Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51-65. doi: 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 19.Bingham SA, Gill C, Welch A, et al. . Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. 1997;26(suppl 1):S137-S151. doi: 10.1093/ije/26.suppl_1.S137 [DOI] [PubMed] [Google Scholar]
- 20.Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009-1018. doi: 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Commission on the Future of Health and Social Care in England The UK private health market: 2014. https://www.kingsfund.org.uk/sites/default/files/media/commission-appendix-uk-private-health-market.pdf. Accessed February 8, 2019.
- 22.Sommerlad A, Perera G, Singh-Manoux A, Lewis G, Stewart R, Livingston G. Accuracy of general hospital dementia diagnoses in England: sensitivity, specificity, and predictors of diagnostic accuracy 2008-2016. Alzheimers Dement. 2018;14(7):933-943. doi: 10.1016/j.jalz.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heim AW. AH 4 Group Test of General Intelligence ASE. Windsor, England: NFER-Nelson Publishing Co LTD; 1970. [Google Scholar]
- 24.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135-140. doi: 10.1016/0028-3932(67)90015-2 [DOI] [Google Scholar]
- 25.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75(12):1070-1078. doi: 10.1212/WNL.0b013e3181f39adc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 27.Solfrizzi V, Custodero C, Lozupone M, et al. . Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer’s disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. 2017;59(3):815-849. doi: 10.3233/JAD-170248 [DOI] [PubMed] [Google Scholar]
- 28.Haring B, Wu C, Mossavar-Rahmani Y, et al. . No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the Women’s Health Initiative memory study. J Acad Nutr Diet. 2016;116(6):921-930.e1. doi: 10.1016/j.jand.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsson E, Karlström B, Kilander L, Byberg L, Cederholm T, Sjögren P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis. 2015;43(1):109-119. doi: 10.3233/JAD-140867 [DOI] [PubMed] [Google Scholar]
- 30.Shatenstein B, Ferland G, Belleville S, et al. . Diet quality and cognition among older adults from the NuAge study. Exp Gerontol. 2012;47(5):353-360. doi: 10.1016/j.exger.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 31.Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. Adherence to a Mediterranean-style diet and effects on cognition in adults: a qualitative evaluation and systematic review of longitudinal and prospective trials. Front Nutr. 2016;3:22. doi: 10.3389/fnut.2016.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74(4):580-591. doi: 10.1002/ana.23944 [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. 2017;7:41317. doi: 10.1038/srep41317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baquero M, Martín N. Depressive symptoms in neurodegenerative diseases. World J Clin Cases. 2015;3(8):682-693. doi: 10.12998/wjcc.v3.i8.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner M, Dartigues JF, Samieri C, Proust-Lima C. Modeling risk-factor trajectories when measurement tools change sequentially during follow-up in cohort studies: application to dietary habits in prodromal dementia. Am J Epidemiol. 2018;187(4):845-854. doi: 10.1093/aje/kwx293 [DOI] [PubMed] [Google Scholar]
- 36.Kesse-Guyot E, Andreeva VA, Lassale C, et al. ; SU.VI.MAX 2 Research Group . Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. 2013;97(2):369-376. doi: 10.3945/ajcn.112.047993 [DOI] [PubMed] [Google Scholar]
- 37.Wengreen H, Munger RG, Cutler A, et al. . Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271. doi: 10.3945/ajcn.112.051276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozawa M, Ninomiya T, Ohara T, et al. . Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama study. Am J Clin Nutr. 2013;97(5):1076-1082. doi: 10.3945/ajcn.112.045575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Food Frequency Questionnaire (FFQ) Data Derivation Procedures, Assessment and Categorization of Covariates, and Description of Sensitivity Analyses
eTable 1. Construction and Distribution of AHEI Scores in the 8225 Whitehall II Participants
eTable 2. Food Groups Used for Factor Analyses to Identify Dietary Patterns
eTable 3. Factor Loadings (≥0.40) on two Dietary Patterns Identified Using Principal Component Analysis
eTable 4. Comparison of Characteristics of Participants Included and Excluded From the Analyses
eTable 5. Association Between Dietary Exposures and Incidence of Dementia, Detailed Adjustment Models
eTable 6. Association Between Dietary Exposures and Incidence of Dementia, Additional Adjustment for APOE ɛ4 Genotype
eTable 7. Association Between Mean AHEI Score (1991-1993, 1997-1999, 2002-2004) and Incidence of Dementia (Follow-up From 2002-2004 to 2017; N Cases/ Total N=353/8268)
eTable 8. Association Between Dietary Exposures in 1991-1993 and Cognitive Function in 1997-1999 and 18 Years Later in Fully Adjusted Models (N=6961)
eTable 9. Association Between Dietary Exposures in 1997-1999 and Cognitive Decline From 1997-1999 to 2015-2016 in Fully Adjusted Models
eTable 10. Association Between Dietary Exposures and Incidence of Dementia Using Fine and Gray Model for Competing Risk of Mortality
eTable 11. Association Between Dietary Exposures and Incidence of Dementia With and Without History of Cardiovascular Disease (CVD)
eTable 12. Association Between Dietary Exposures and Incidence of Dementia Using Inverse Probability Weighting to Take Missing Data Into Account
eTable 13. Association Between Dietary Exposures and Incidence of “Possible” Dementia Defined as low Cognitive Performance or Electronic Record of Dementia Diagnosis
eTable 14. Association Between Dietary Exposures and Mortality
eTable 15. Association Between the Mediterranean Diet Score and Incidence of Dementia