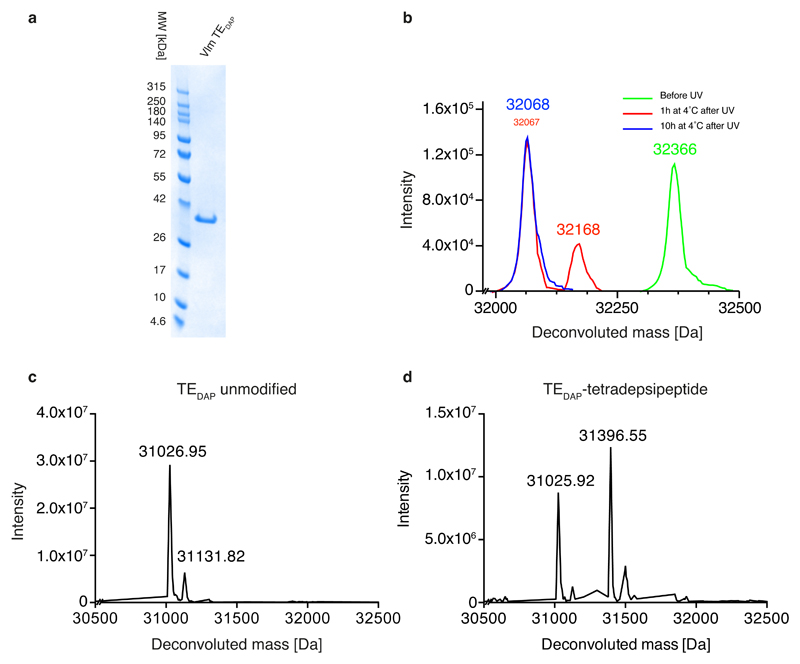
Extended Data Fig. 7. Expression and substrate conjugation to Vlm TE containing DAP at position 2,463.
a, Following expression and purification of Vlm TEDAP, the protein was loaded on an SDS–PAGE gel and Coomassie stained; the experiment was repeated in two biological replicates with similar results. b, The deprotection of 6 in TEDAP–strep was followed by ESI–MS analysis. Green trace, purified TEDAP–strep containing 6 at position 2,463: expected mass 32,364.6 Da, observed 32,365.78 Da. Red trace, TEDAP–strep containing 6 at position 2,463 following illumination to convert 6 to the intermediate: expected 32,171.56 Da, observed 32,168.48 Da; and further incubation (1 h, 4 °C) to convert the intermediate to product: expected 32,067.62 Da, observed 32,068 Da). Blue trace, TEDAP–strep containing 6 at position 2,463 following illumination (to convert 6 to the intermediate) and further incubation (10 h, 4 °C) to convert the intermediate to DAP (1): expected 32,067.62 Da; observed, 32,067.84 Da. The experiment was repeated in two biological replicates with similar results. c, Purified TEDAP after illumination and intermediate fragmentation: expected 31,027.24 Da, observed 31,026.95 Da and 31,131.82 Da. d, TEDAP incubated with tetradepsipeptidyl–SNAC 7: expected 31,027.24 Da (unmodified) and 31,398.69 Da (modified); observed 31,025.92 Da and 31,396.55 Da. The experiments in panels c, d were repeated independently two times with similar results.