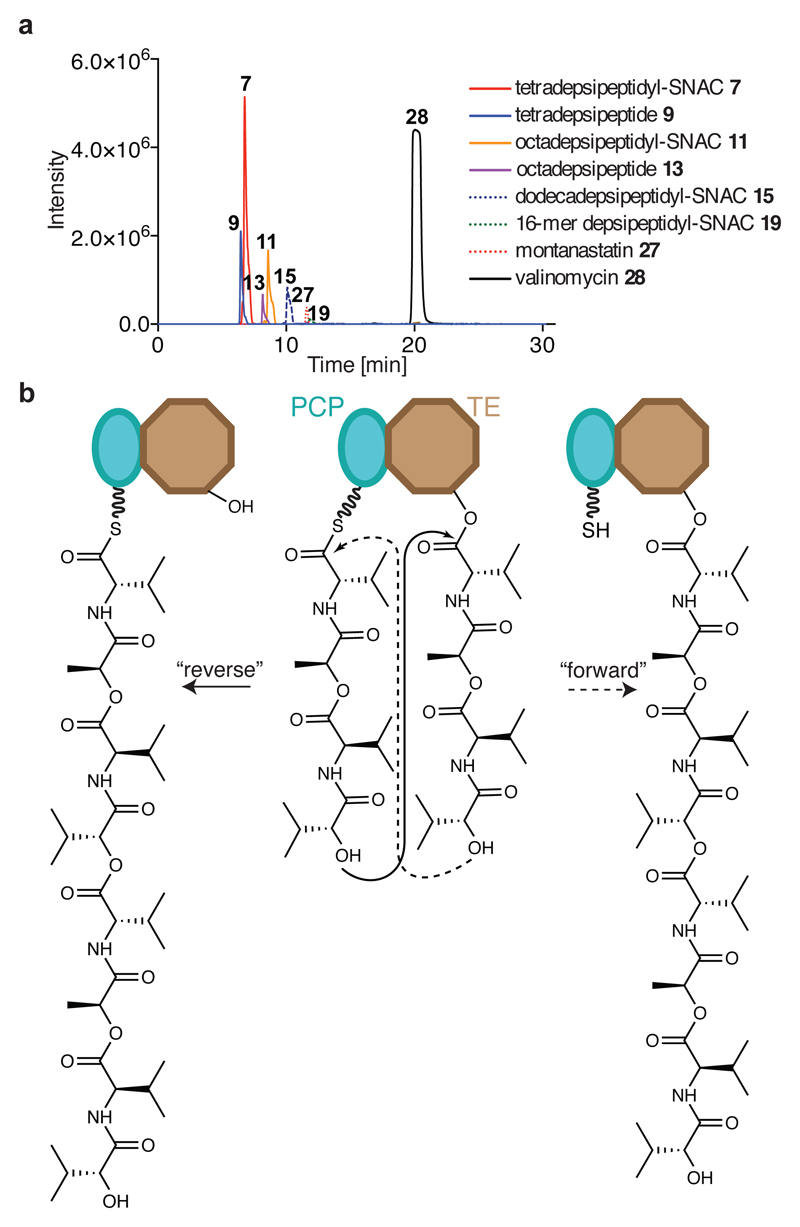
Fig. 3. Vlm TE produces valinomycin and intermediates that delineate the oligomerization pathway from tetradepsipeptidyl–SNAC.
a, Extracted ion chromatograms (EICs) from high-resolution (HR)–liquid chromatography (LC)–ESI–MS of reactions of tetradepsipeptidyl–SNAC (7; 1.7 mM) and Vlm TE (6.5 μM); TEwt produces valinomycin as its major product. The experiment was performed two independent times with similar results. See ‘Supplementary Methods for Statistics and Reproducibility’ for mass analysis and deviations from calculated m/z values. b, Two scenarios for oligomerization17,26. In the ‘forward transfer’ scenario, the distal hydroxyl group of tetradepsipeptidyl–O-TE (TE) attacks (dotted line) the thioester group in tetradepsipeptidyl–S-PCP (PCP), directly forming octadepsipeptidyl–O-TE (right). In the ‘reverse’ scenario, the distal hydroxyl group of tetradepsipeptidyl–S-PCP attacks the ester group in tetradepsipeptidyl–O-TE, forming octadepsipeptidyl–S-PCP (left), which would later be transferred onto the TE domain serine. Our data are consistent with the ‘reverse’ oligomerization scenario; see also Extended Data Fig. 5.