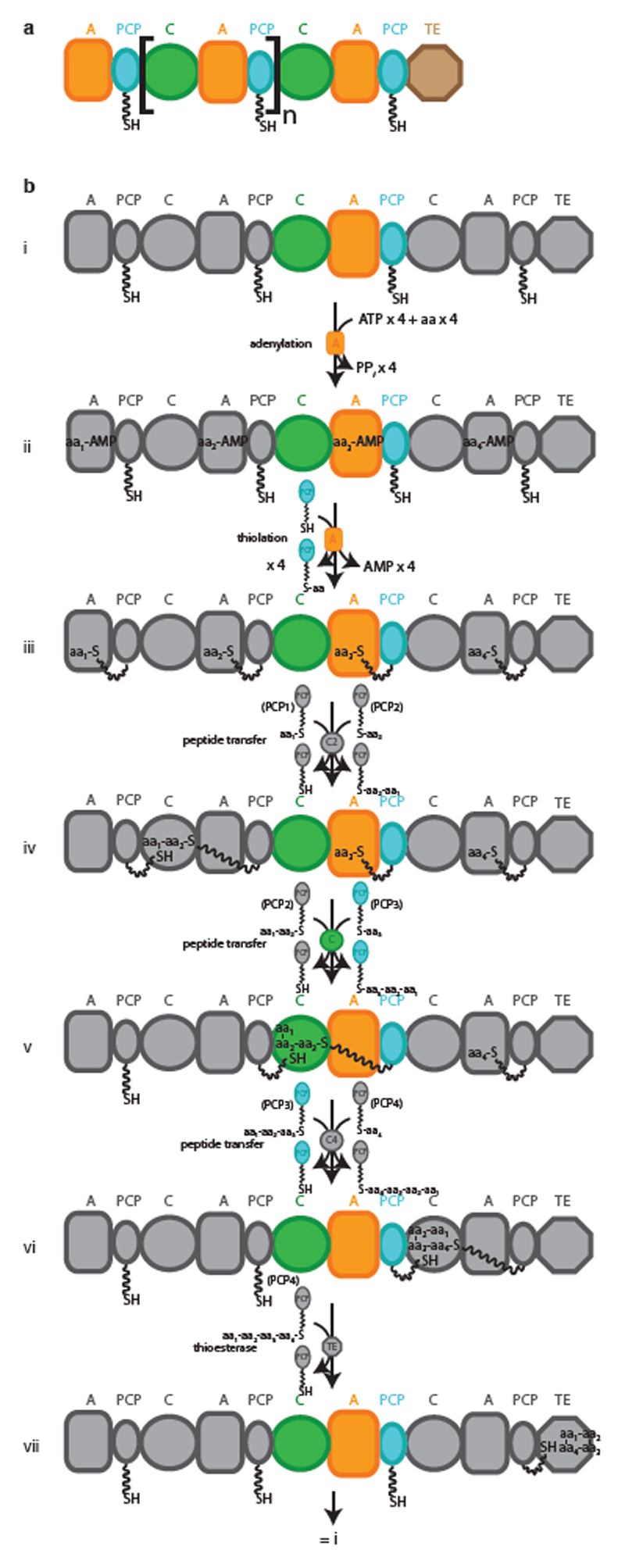
Extended Data Fig. 1. Schematic representation and reaction cycle of a canonical NRPS.
a, Schematic representation of a generic type I NRPS. The square brackets denote a single module. b, i–vii, Synthetic cycle of a canonical elongation module. NRPSs assemble peptides from amino acyl and other small acyl building blocks using a modular and thio-templated logic. A canonical NRPS is composed of one module for every residue in the peptide product. The initiation module contains an adenylation (A) domain, which binds cognate acyl substrate and performs adenylation and transfer of that substrate as a thioester on the phosphopantetheine arm (PPE, shown as a wavy line) of a peptidyl carrier protein (PCP) domain, for transport between active sites. Each elongation module contains an A and a PCP domain, and also a condensation (C) domain, which condenses aminoacyl and peptidyl substrates bound to PCP domains, thus progressively elongating the nascent chain. Termination modules contain C, A and PCP domains, and a specialized terminating/offloading domain responsible for the release of the peptide in its final form. The most common and most versatile terminating domain in NRPSs is the TE domain. Similar TE domains terminate synthesis in polyketide and fatty acid synthases. PPi, diphosphate; aa, amino acid.