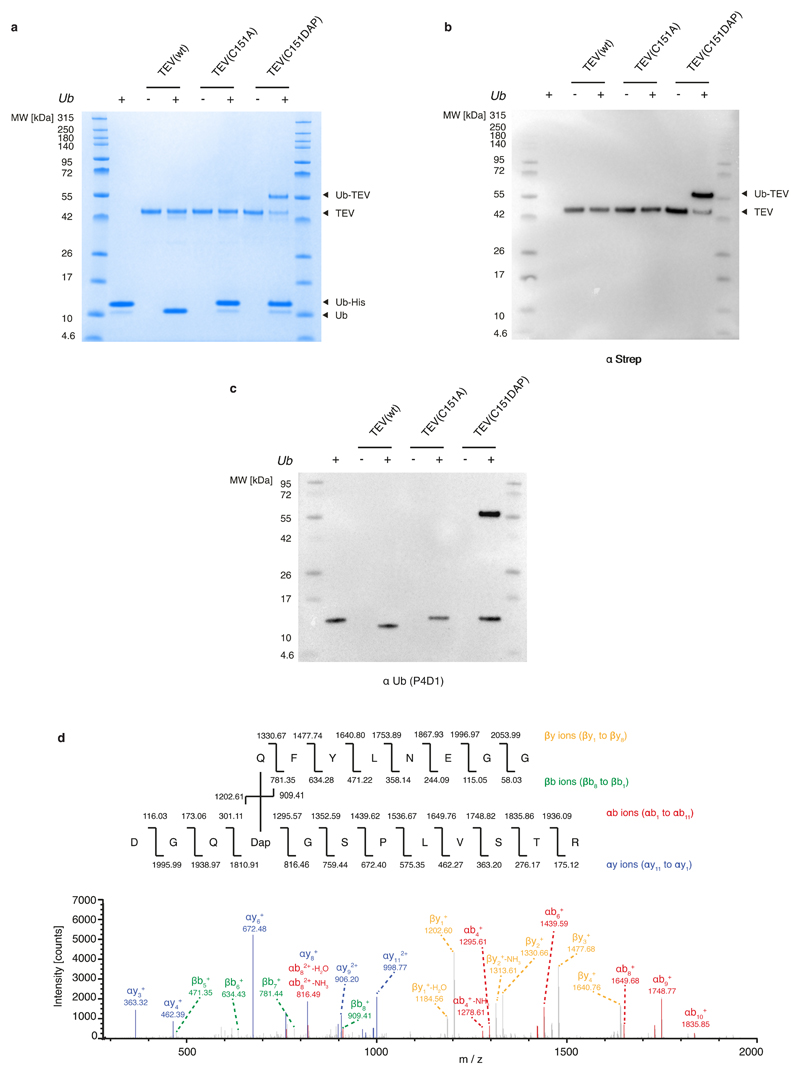
Extended Data Fig. 3. Stably trapping the acyl–enzyme intermediate of a cysteine protease.
a, Different variants of TEV protease (shown at the top) were reacted with Ub–tev–His. The use of TEV(wt) results in cleavage of the TEV cleavage sequence. The use of TEV(C151A) results in minimal cleavage. The presence of DAP in the active site of TEV results in the presence of an extra band in the Coomassie gel, representing the isopeptide-linked TEV(C151DAP)–Ub complex. b, c, Anti-streptavidin (α-strep; b) and anti-Ub (α-Ub antibody P4D1; c) western blots of the reactions confirm the identity of the complex. For panels a–c, the experiment was repeated in two biological replicates with similar results. d, Tandem mass spectrometry following tryptic digest of the TEV(C151DAP)–Ub conjugate confirms amide-bond formation at the expected position. Top, the sequence of the branched peptide subject to fragmentation. Fragmentation of the substrate chain is predicted to lead to a series of y ions (yellow) and a series of b ions (green); the ions from this chain are labelled as ‘β’. Fragmentation of the TEV(C151DAP)-derived chain is predicted to lead to a series of y ions (blue) and a series of b ions (red); the ions from this chain are labelled as ‘α’. Bottom, MS/MS spectra with peak assignments. Ions in the α-chain were assigned by treating DAP and the β-chain as a modification of known mass. Ions in the β-chain were manually assigned. The mass-spectrometry analysis was performed once.