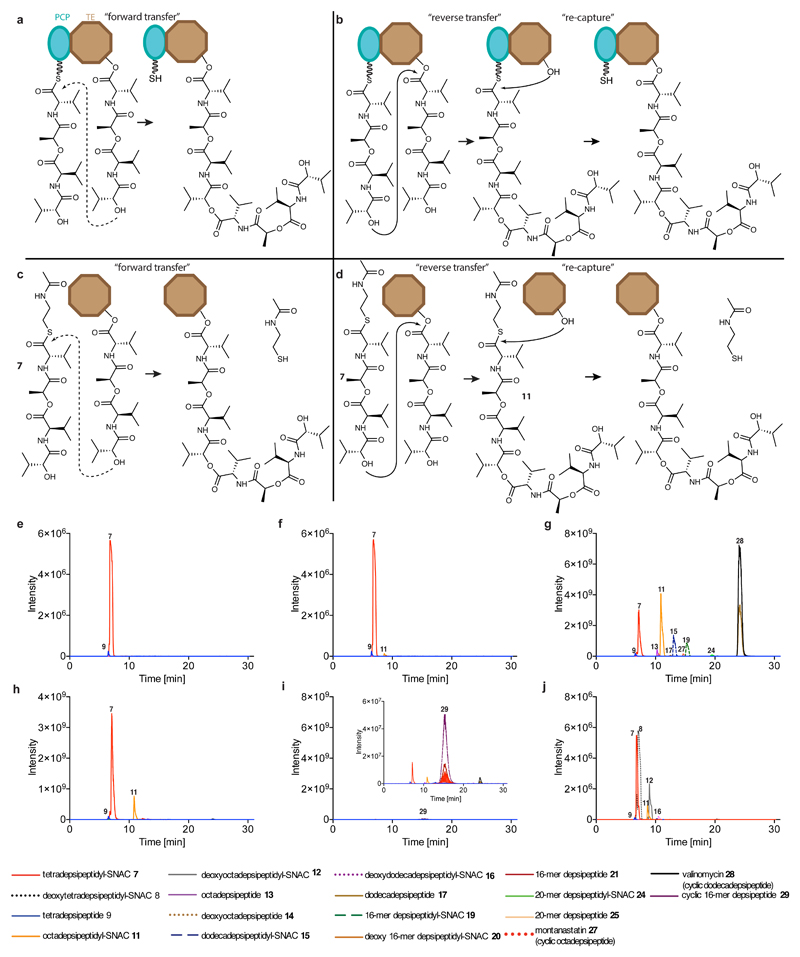
Extended Data Fig. 5. The mechanism by which by Vlm TE catalyses oligomerization.
Oligomerization could conceivably take place in two ways. a, In the first scenario, ‘forward transfer’, the distal hydroxyl group of the tetradepsipeptidyl–O-TE complex attacks the thioester group in the tetradepsipeptidyl–S-PCP enzyme intermediate, directly forming octadepsipeptidyl–O-TE as a product. b, In the second scenario, ‘reverse transfer’, the distal hydroxyl group of the tetradepsipeptidyl–S-PCP complex attacks the ester group in the tetradepsipeptidyl–O-TE enzyme intermediate, forming octadepsipeptidyl–S-PCP as a product, which would then need to be transferred onto the TE-domain serine (here labelled as ‘re-capture’). c, d, Analogous scenarios involving tetradepsipeptidyl–SNAC (7) as the substrate instead of tetradepsipeptidyl–S-PCP. e, f, EICs (HR LC–ESI–MS) of a mix of 7 (1.7 mM) and buffer (e), or the products of a reaction between 7 (1.7 mM) and Vlm TEDAP (6.5 μM) (f). g–i, EICs (low-resolution (LR) LC–ESI–MS) of reactions using a higher-volume injection into an ion-trap MS instrument. g, The higher-volume injection of a reaction of 7 (1.7 mM) and Vlm TEwt (6.5 μM) enabled detection of a peak consistent with the 20-mer depsipeptidyl–SNAC (24). h, LC-Ion-trap MS of reaction of 7 (1.7 mM) and Vlm TEDAP (6.5 μM). i, Small amounts of the cyclic 16-mer depsipeptide 29 elute during post-run column clean-up of experiment shown in g. j, EICs (HR LC–ESI–MS) of products of reactions between Vlm TEwt (6.5 μM) and a mix of 7 and deoxy-tetradepsipeptidyl–SNAC (8; 1.7 mM of each). TEwt produces the intermediates deoxy-octadepsipeptidyl–SNAC (12), deoxy-dodecadepsipeptidyl–SNAC (16) and deoxy 16-mer depsipeptidyl–SNAC (20), confirming the reaction pathway shown in panel b. See ‘Supplementary Methods for Statistics and Reproducibility’ for accurate mass analysis and deviations from calculated m/z values of each compound. The experiments in panels e–i were repeated independently two times with similar results. Mass-spectrometry analysis of the experiment in panel j was performed once.