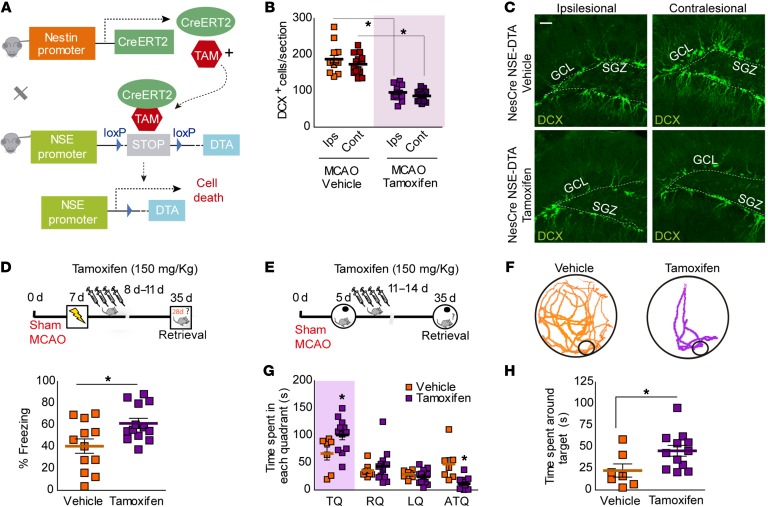
Figure 7. Conditional deletion of newborn neurons after stroke decreases cognitive deficits.
(A) Genetic strategy used for conditional deletion of newborn neurons after stroke. (B) Number of DCX+ cells in MCAO Nestin-CreERT2/NSE-DTA mice treated with vehicle or tamoxifen. Two-way ANOVA showed a significant effect of tamoxifen in the number of DCX+ cells (F(1,48) = 136.59; P < 0.0001) in both ipsi- and contralesional sides (Bonferroni’s post hoc: *P < 0.05 vs. Nes-CreERT2/NSE-DTA vehicle; vehicle, n = 14; tamoxifen, n = 12). (C) Representative images of DCX+ cells (green) in ischemic Nestin-CreERT2/NSE-DTA mice after vehicle or tamoxifen 35 days after surgery. Scale bar: 30 μm. (D) Percentage of freezing in vehicle- or tamoxifen-Nestin-CreERT2/NSE-DTA treated mice 35 days after CFC (*P < 0.05 vs. Nestin-CreERT2/NSE-DTA vehicle; vehicle, n = 12 and tamoxifen, n = 13). Upper panel: experimental design. Seven days after ischemia, Nestin-CreERT2/NSE-DTA mice were subjected to CFC (0.6 mA × 3), and 24 hours later, they were treated i.p. with vehicle or tamoxifen (150 mg/kg) over 4 consecutive days and tested in the CFC 35 days after surgery. (E–H) Experimental design for assessing spatial memory retention in the Barnes maze (E). Five days after ischemia, Nestin-CreERT2/NSE-DTA mice were trained during 7 days (3 session/d) in the Barnes maze. After training, mice were treated with vehicle or tamoxifen and tested 28 days later. (F) Representative traces during the retention test. (G) Time spent in each quadrant of the Barnes maze platform. Two-way ANOVA demonstrated a significant interaction between quadrants and tamoxifen treatment in ischemic Nestin-CreERT2/NSE-DTA (F(3,68) = 4.41; P = 0.0068; Bonferroni’s post hoc: *P < 0.05 vs. Nestin-CreERT2/NSE-DTA vehicle TQ). (H) Time spent around target hole during testing (*P < 0.05 vs. MCAO Nestin-CreERT2/NSE-DTA vehicle; vehicle, n = 7; tamoxifen, n = 12). Data are represented as mean ± SEM. Data were compared by using nonparametric 2-tailed Mann-Whitney U tests (D and H) and nonparametric 2-way ANOVA followed by Bonferroni’s post hoc testing (B and G).