Figure 1. The MFN-mediated fusion models.
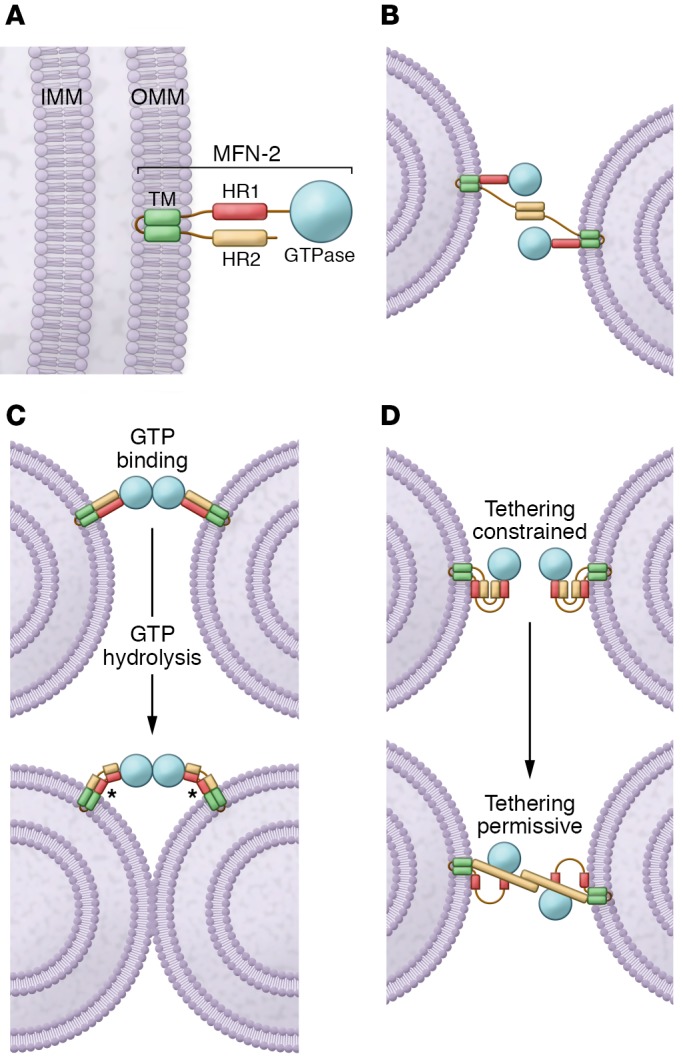
(A) Schematic of the MFN2 protein. MFN2 is embedded in the OMM by a TM and is composed of a GTPase domain and heptad repeat domains (HR1 and HR2). IMM, inner mitochondrial membrane. (B) MFN2 interacts in trans forming either homotypic or heterotypic (with MFN1) dimers to produce the mitochondrial tethering that precedes mitochondrial fusion. (C) MFNs may dimerize in trans upon GTP binding, which leads to long-distance docking of mitochondria. GTP hydrolysis can then induce a large conformational rearrangement of MFNs that brings OMMs into closer proximity. Mitochondrial fusion proceeds as a result of local membrane deformation near the TM domain when MFNs undergo GTP hydrolysis–dependent conformational transition and membrane structure perturbation by the HR1 domain (asterisks). (D) Schematic depiction of the folded (with HR2 constrained; left) and unfolded (with HR2 extended; right) MFN conformations. HR2 unfolding is below.
