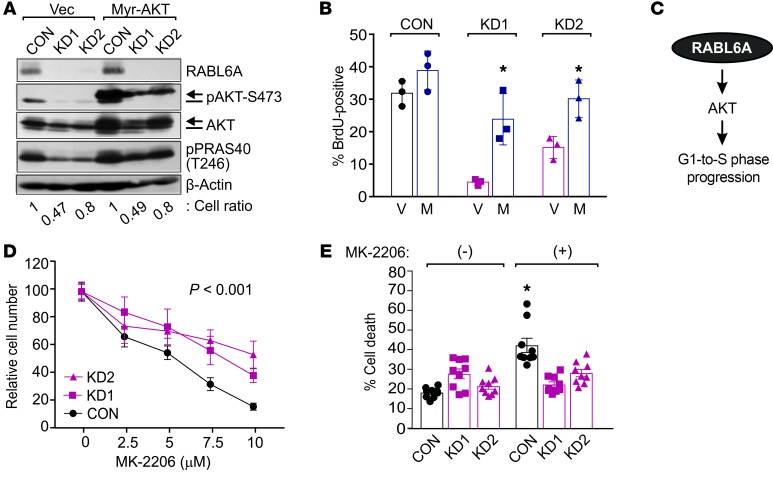
Figure 2. RABL6A-AKT signaling is required for PNET cell cycle progression and response to AKT inhibitors.
(A) BON-1 cells expressing vector (Vec) or constitutively activated myristoylated AKT (Myr-AKT) were infected with control (CON) or RABL6A shRNAs (KD1, KD2). Representative Western blots of phosphorylated AKT-S473 detect endogenous AKT (bar, lower band) and Myr-AKT (arrow, upper band only in lanes 4-6). Heightened activity of Myr-AKT was assessed by levels of PRAS40-T246 phosphorylation. β-actin served as the loading control. Cell ratios (i.e., relative cell numbers normalized to CON cells) are indicated for each sample. (B) The percentage of BrdU-positive cells was quantified in BON-1 control and RABL6A knockdown cells expressing vector (V) versus Myr-AKT (M). Data were quantified from 3 independent experiments; *P < 0.02 for V versus M comparison, 2-way ANOVA. (C) Schematic of RABL6A promoting G1-to-S phase progression and PNET cell proliferation by activating AKT signaling. (D) Dose response curves, shown as relative cell number, in BON-1 control and RABL6A knockdown cells treated for 5 days with increasing concentrations of the AKT inhibitor, MK-2206. Data represent the mean ± SEM for triplicate samples from 3 separate experiments, in which results were normalized to values for untreated cells within each group. *P < 0.001 for KD1 or KD2 compared with CON, 2-way ANOVA and adjusted for multiple comparisons using the Bonferroni method. Overall differences between the curves were assessed by generalized linear regressions. (E) Percentage of cell death in BON-1 control and RABL6A knockdown cells following treatment with MK-2206 (10 μM) for 3 days. Data represent the mean ± SEM from 3 independent experiments. *P < 0.001 for (+) versus (–) comparison, 2-way ANOVA and adjusted for multiple comparisons using the Bonferroni method.