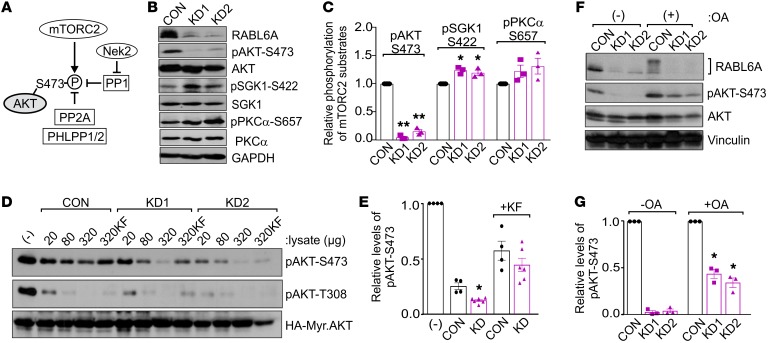
Figure 3. RABL6A increases AKT-S473 phosphorylation through inhibition of PP2A.
(A) Schematic of the kinases and phosphatases that regulate AKT-S473 phosphorylation. P, phosphorylation; arrows, activating events; perpendicular bars, inhibiting events. (B) Western blots of BON-1 control (CON) and RABL6A knockdown (KD1 and KD2) cells showing selective loss of pAKT-S473 in RABL6A-depleted cells versus moderately increased phosphorylation of other mTORC2 substrates, SGK1 and PKCα. GAPDH was the loading control. (C) Relative phosphorylation of mTORC2 substrates in BON-1 CON, KD1, and KD2 cells was quantified by ImageJ. Data represent the mean ± SD from 3 independent experiments (*P < 0.05 and **P < 0.001 compared with CON, 2-way ANOVA and adjusted for multiple comparisons using the Bonferroni method). (D) Western blots of pAKT-S473 and pAKT-T308 following in vitro phosphatase assays using phosphorylated HA-tagged Myr-AKT as substrate to which the indicated amounts (μg) of BON-1 CON, KD1, or KD2 lysates were added. As controls, buffer (–) or the general phosphatase inhibitor potassium fluoride (KF), was added to substrate prior to the phosphatase reaction. (E) Relative levels of pAKT-S473, normalized to total HA-Myr-Akt, were quantified by ImageJ analysis of blots from 3 or more experiments in which 320 μg cell lysate was tested. *P < 0.005 KD (KD1 and KD2) versus CON, 2-way ANOVA adjusted for multiple comparisons using the Bonferroni test. (F) Western blots of pAKT-S473, AKT, and RABL6A following okadaic acid (OA) treatment (100 nM, 20 hours) in BON-1 CON and KD cells showing significant restoration of pAKT-S473 by PP2A inhibition. Vinculin was loading control. (G) Relative phosphorylation of AKT-S473 was quantified from 3 experiments. *P < 0.005 compared with untreated counterparts, 2-way ANOVA adjusted for multiple comparisons using the Bonferroni method. Western blots in B, D, and F are representative of 3 or more experiments.