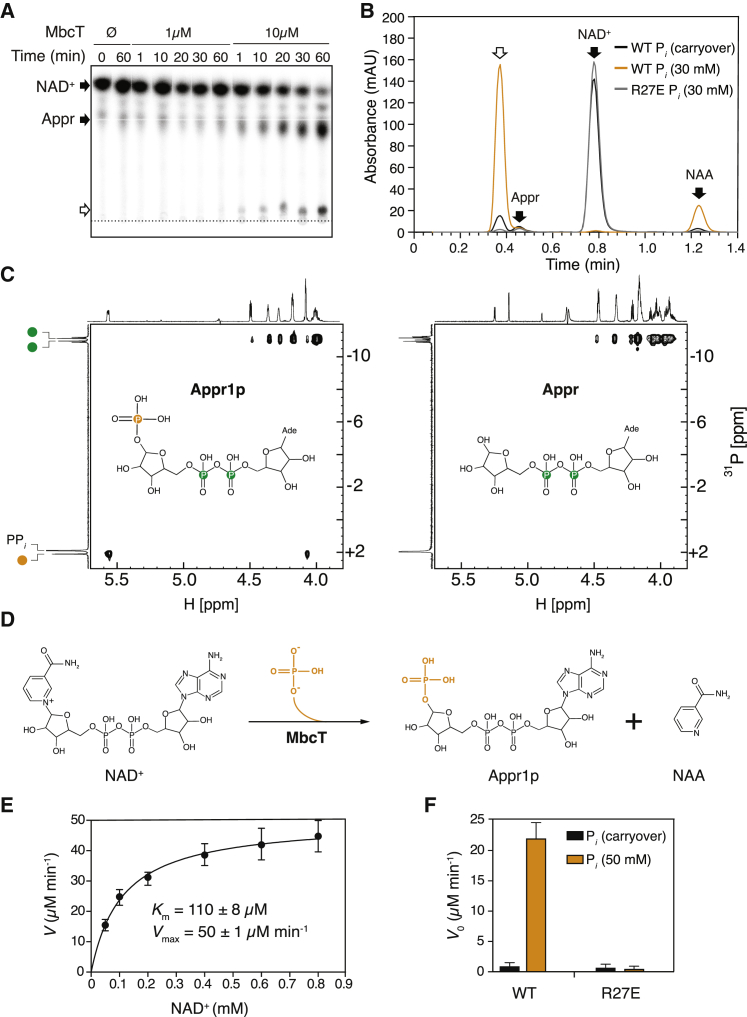
Figure 3.
Enzymatic Activity of MbcT
(A) Representative autoradiograph of a TLC plate showing MbcT-mediated depletion of 32P-NAD+ over time and simultaneous accumulation of 32P-ADP-ribose (Appr) and a secondary reaction product (white arrow). The dotted line indicates the position where samples were applied to the plate. Similar results were obtained in two independent experiments.
(B) Representative HPLC chromatograms of the reaction products of NAD+ (0.5 mM) with MbcT (0.7 μM) in the presence (orange) or absence (black) of sodium phosphate (30 mM). The white arrow indicates the reaction product formed in addition to nicotinamide (NAA) (cf. A). MbcT-R27E does not degrade NAD+ in sodium phosphate (30 mM) buffer (gray line). NAD+, Appr and NAA were identified by retention time comparisons with standards. The observed Appr is an impurity found in the commercial substrate. Similar results were obtained in three independent experiments.
(C) 1H-31P HSQC31 spectra of the reaction products of NAD+ (5 mM) with MbcT (10 mM) and, for reference, of pure Appr (5 mM). Phosphate atoms from the ADP-ribose moiety are colored green, whereas the phosphate atom derived from orthophosphate is highlighted in orange.
(D) Proposed reaction mechanism of MbcT-mediated NAD+ phosphorolysis yielding ADP-ribose-1″-phosphate (Appr1p) and NAA.
(E) Kinetics of NAD+ phosphorolysis by MbcT (50 nM). Km and Vmax values were determined by nonlinear regression analysis with the Michaelis-Menten equation.
(F) Comparison of initial velocity (V0) of NAD+ phosphorolysis of WT MbcT (50 nM) and MbcT-R27E (50 nM) in the presence or absence of sodium phosphate (50 mM). The initial velocities were determined at a substrate concentration of 100 μM. For data in (E) and (F), data are represented as mean of eight and four independent replicates ± SD, respectively.
See also Figures S5 and S6.