Figure 6.
The FG Motif Contributes to Dimerization of Several Importins
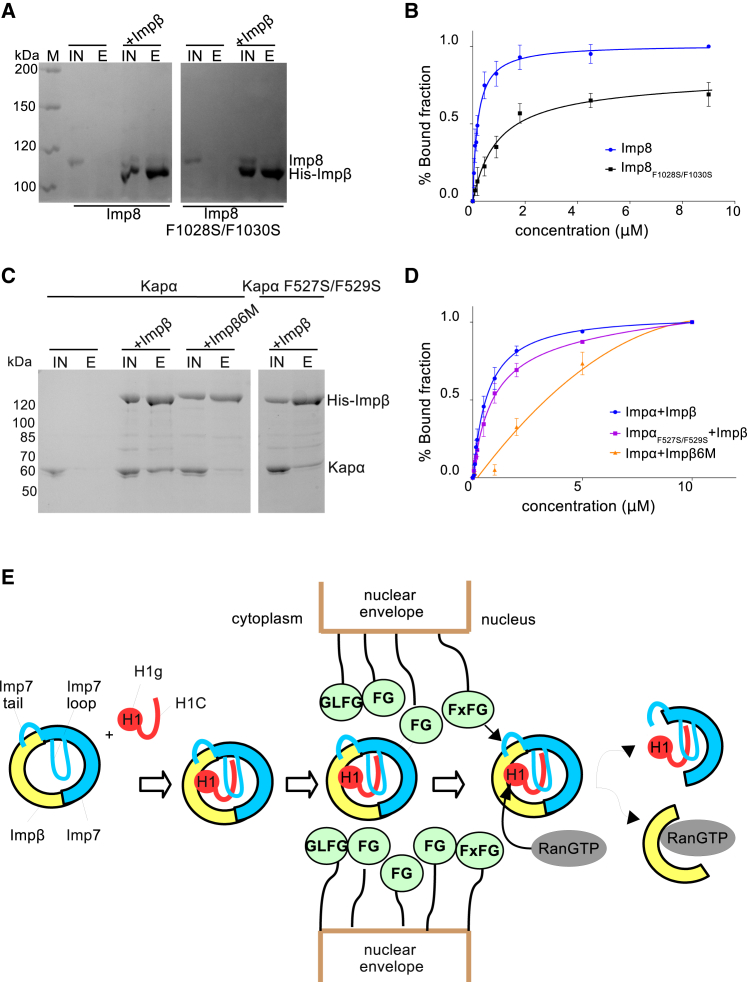
(A) Results of a pull-down experiment showing that conserved phenylalanines in the Imp8 C-terminal tail are required for the interaction with Impβ. Shown is SDS-PAGE analysis of IN and E fractions of the pull-down experiment, with wild-type or mutant Imp8 binding to His-tagged wild-type Impβ.
(B) Quantification of the results of three independent gel shift experiments, showing Imp8 and Imp8 mutant binding to Impβ. The Kd was estimated from the binding curve. The Kd is 0.19 μM for wild-type Imp8 and 1.72 μM for Imp8 F1028S and F1030S.
(C) Results of a pull-down experiment showing that conserved phenylalanines in the Impα C-terminal tail (Impα F527S/F529S) contribute to the interaction with Impβ. Likewise, the mutations in the FxFG binding pocket of Impβ, Impβ6M, destabilize the interaction with wild-type Impα. Shown is SDS-PAGE analysis of IN and E fractions of the pull-down experiment with His-tagged wild-type and mutant Impβ.
(D) Quantification of the results of three independent gel shift experiments showing Impα and Impα mutant binding to Impβ and the Impβ6M mutant. The Kd was estimated from the binding curve. The Kd is 0.62 μM for wild-type Impα and 0.92 μM for Impα F527S/F529S. The Kd for Impα binding to Impβ6M is 3.34 μM.
(E) In the first step, the FG motif in the Imp7 C-terminal tail binds to Impβ, mimicking FG-nucleoporin interactions. The affinity of this complex for H1 in the cytoplasm is higher than that of individual importins because the complex can efficiently accommodate its charged C-terminal tail in the cradle and between the HEAT repeat motifs. At the same time, the disordered C-terminal tail of H1 stabilizes and shapes the complex structure. The Imp7:Impβ:H1 complex translocates through the NPC mainly via transient FG-nucleoporin:importin interactions. At the nuclear side of the NPC, Impβ dissociates from H1 by RanGTP binding and by competing FxFG-nucleoporin interactions. H1 is then transferred to nuclear chaperones.
See also Figure S7.