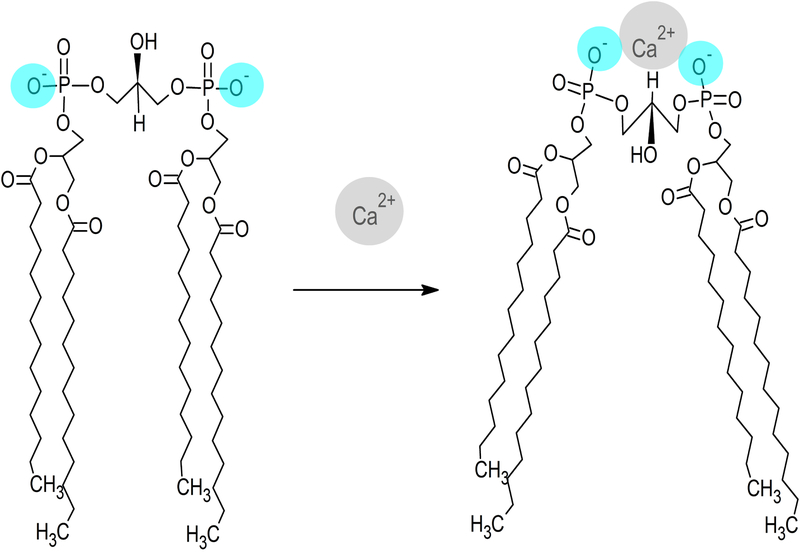
Figure 7. Model depicting the effect of calcium binding on CL structure.
Divalent cations, such as Ca2+, are attracted to the anionic polar head group of CL, forming a bidentate binding interaction with its two phosphate moieties (blue). A single calcium ion localized between these phosphates induces structural repositioning of CL in order to maximize interaction with calcium. As the phosphate moieties converge toward the calcium ion, conformational strain is relieved by realignment of the CL fatty acyl chains, which results in the adoption of a conformation that is incompatible with a bilayer state.