Figure 1.
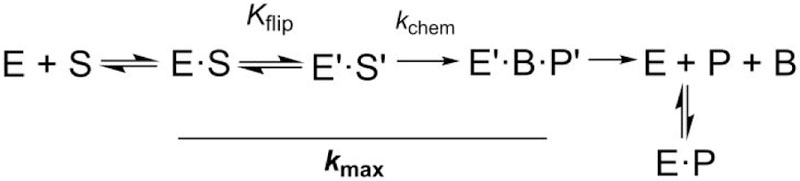
Minimal kinetic mechanism for the TDG reaction. Association of enzyme (E) and DNA substrate (S) gives an initial collision complex (E·S), then nucleotide flipping (Kflip) and potentially other conformational changes give the reactive enzyme-substrate complex (E′·S′). In the chemical step (kchem), cleavage of the N-glycosyl bond and addition of the nucleophile (water) generate the ternary product complex (E′·B·P′, where B is the excised base and P is abasic DNA). Dissociation of E′·B·P′ likely involves rapid release of B and slow release of abasic DNA. The solid line denotes reaction steps that contribute to kmax, the rate constant obtained from the single turnover kinetics experiments.
